上幾個星期化學友同幾個學生去左香島中學睇一年一度嘅化學奧林匹克(Chemistry Olympiad)比實,除左去學吓o野同埋觀摩吓各隊表現之外,順便都見o下d幾個月冇見嘅老友。
今年嘅主題係chemistry in commercial products。決賽Present嘅六組同學都搵咗d唔同嘅commercial products去分析同研究: 螢光棒、染髮劑、洗衣液、解酒藥、螺旋藻片同埋驅鳥劑。
講真,六組同學嘅表現幾出色,見到佢哋都花咗唔少心機去準備,係一場高質素嘅比賽。化學友唔想o向e度慢慢講d關於o個日化學嘅內容,反而化學友想講講o個日嘅冠軍St. Paul’s Convent School。其實o個日未宣報邊個係冠軍,化學友都100%肯定St Paul會得到champion o架啦。佢地o個日嘅表現可以話接近完美,勝出係實至名歸。如果同學仔有參加o個以前d chemistry或者science嘅competitions,都會知道St Paul係常客,而每次佢地都會有好好嘅表現。一次成功仲可以話係luck,但每次都有高水準表現就一定係有佢地成功之處。化學友發覺最明顯St Paul比其他學校好嘅地方係佢地每次比賽都可以做到”以終為始”,而一般學校好多時都做唔到。同學仔可能會問乜o野叫做"以終為始" ? 簡單o黎講就係每決定做一件事時,第一樣o野要做就係要定下明確目標先,然後所有過程都係向住e o個目標出發。用St Paul為例,佢地o個project係compare染髮劑,而佢地做嘅每一個experiment或者investigation都係有明確咁為左compare染髮劑而做,唔會左右搖擺。反觀其他學校或多或少都有d守唔住目標,佢地好多時都係為左做實驗而做實驗,而o個d實驗同佢地嘅target係冇乜關係,當d judge問佢地為乜做e o個實驗,同常佢地都唔係好知點解。就係因為有e o個分別,好多時St Paul參加competition都會欏到好成績。老土d咁講,每個人成功都一定有佢嘅優勝地方,絕非僥幸o架。化學友希望同學仔以後參加任可science competition都一定要記住”以終為始”,咁樣你地得到好成績嘅機會就大好多o架啦。(P.S. 同學仔唔好以為化學友o向到同St. Paul賣告白,化學友同St. Paul一d關係都冇o架,只不過化學友真係幾欣賞佢地嘅表現,覺得同學仔參加competition時可向佢地借鏡咋。)
有興趣睇吓Chemistry Olympiad嘅同學仔,可以去e個website睇睇丫:
http://www.hkasme.org 搵 ”HKChO-12th”
2007年5月13日 星期日
2007年5月12日 星期六
曼聯----2007 champion
Go!Go!Go! 曼聯終於欏到聯賽冠軍啦!!!化學友支持左man utd16,7年o架喇,e幾年man utd嘅performance真係令化學友有d失望。但係今年化學友終於可以再次見到曼聯奮力一戰嘅表現,令到本來輸嘅比賽變左打和,和嘅比賽變左贏,好touch呀。講真,化學友覺得今年曼聯嘅overall實力係及唔上車仔,liverpool,甚至arsenal,但係佢嘅鬥志加上少少幸運所以先win到chmapion。希望o黎緊費sir買多d好嘅球員喇,唔好再亂買人喇!!!!!!
Yeah! 曼聯必勝!!!!!!
Yeah! 曼聯必勝!!!!!!
回應
回應1
Pinky:
你好,化學友。
我想請問一下如何才能學好chem,
我是指在CE 摘B OR A。
駛唔駛睇多D課外書架CHEM?
化學友:
pinky,你條問題幾難答呀,因為各施各法,好難一概而論。不過化學友發覺自己大部份欏A嘅學生都有一個共通點-----佢地都做得幾多exercise。因為好多時你讀完一d concept,以為自己好明,但係一做exercise,先知自己唔識用o架。多做exercise真係好有用o架,可以令到你溫習事半功倍。坦白講,化學友dF5學生平均每年大約有30% chem欏A,而化學友覺得佢地唔係個個都好聰明,但係好多都係做得exercise多所以先欏到A。化學友深信"practice makes perfect",Pinky你可以試o下e個方法對你有冇幫助呀。
回應2
Wa:
化學友你好...
i would like to ask u one qu abt the chemical reaction in the lemon cell (silver / copper)
1. will copper be dissolved , ie oxidised?...
2. wt would happen if we use nacl soln instead of lemon...?
thx!!!
化學友:
1. Wa, 因為Cu is more reactive than Ag,所以o向lemon cell裡面Cu會lose electron而形成Cu2+:
Cu(s)--> Cu2+(aq) + 2e-
因此,copper會dissolves而變成Cu2+(aq)。仲有因為Cu嘅oxidation number由0變成+2,所以Cu is oxidized。不過化學友覺得你可能未學乜o野叫oxidation number,暫時黎講你可以當一隻element嘅charge增加時(e.g. Cu --> Cu2+),隻element就oxidized,但係當一隻element嘅charge減少時(e.g. Cu2+--> Cu),隻element就reduced。
2. 當NaCl instaed of lemon時,Cu一樣係會dissolve形式Cu2+,因為無論用乜o黎做electrolyte,都唔會影o向anode放電子嘅現象,Cu點都會變成Cu2+。而H2就會o向Ag到形成(至於explanation,你呀sir/miss遲d會教o架啦)。
回應3
ag925:
化學友你好...
i would like to ask u some qus abt a chemical cell using cu/ag as electrodes ...
1. if we use lemon juice as electrolyte ... will copper be oxidized ..?...wt would happen if we used nacl soln as electrolyte...?
化學友:
哈哈.....ag925 你條問題同上面嘅Wa一模一樣o架。你地係唔係同一個人o黎o架?定係你地讀同一間學校??? ag925,如果你唔明o個answer再問化學友丫。
Pinky:
你好,化學友。
我想請問一下如何才能學好chem,
我是指在CE 摘B OR A。
駛唔駛睇多D課外書架CHEM?
化學友:
pinky,你條問題幾難答呀,因為各施各法,好難一概而論。不過化學友發覺自己大部份欏A嘅學生都有一個共通點-----佢地都做得幾多exercise。因為好多時你讀完一d concept,以為自己好明,但係一做exercise,先知自己唔識用o架。多做exercise真係好有用o架,可以令到你溫習事半功倍。坦白講,化學友dF5學生平均每年大約有30% chem欏A,而化學友覺得佢地唔係個個都好聰明,但係好多都係做得exercise多所以先欏到A。化學友深信"practice makes perfect",Pinky你可以試o下e個方法對你有冇幫助呀。
回應2
Wa:
化學友你好...
i would like to ask u one qu abt the chemical reaction in the lemon cell (silver / copper)
1. will copper be dissolved , ie oxidised?...
2. wt would happen if we use nacl soln instead of lemon...?
thx!!!
化學友:
1. Wa, 因為Cu is more reactive than Ag,所以o向lemon cell裡面Cu會lose electron而形成Cu2+:
Cu(s)--> Cu2+(aq) + 2e-
因此,copper會dissolves而變成Cu2+(aq)。仲有因為Cu嘅oxidation number由0變成+2,所以Cu is oxidized。不過化學友覺得你可能未學乜o野叫oxidation number,暫時黎講你可以當一隻element嘅charge增加時(e.g. Cu --> Cu2+),隻element就oxidized,但係當一隻element嘅charge減少時(e.g. Cu2+--> Cu),隻element就reduced。
2. 當NaCl instaed of lemon時,Cu一樣係會dissolve形式Cu2+,因為無論用乜o黎做electrolyte,都唔會影o向anode放電子嘅現象,Cu點都會變成Cu2+。而H2就會o向Ag到形成(至於explanation,你呀sir/miss遲d會教o架啦)。
回應3
ag925:
化學友你好...
i would like to ask u some qus abt a chemical cell using cu/ag as electrodes ...
1. if we use lemon juice as electrolyte ... will copper be oxidized ..?...wt would happen if we used nacl soln as electrolyte...?
化學友:
哈哈.....ag925 你條問題同上面嘅Wa一模一樣o架。你地係唔係同一個人o黎o架?定係你地讀同一間學校??? ag925,如果你唔明o個answer再問化學友丫。
2007年5月7日 星期一
回應
回應2
Schoolbag:
有條題我想問xIO3- + yI- + 6H+ ----->_I2 + _H2O
ANSWER:X=1 Y=5
我想知Y可吳可以系3?點解?
化學友:
schoolbag,化學友唸你當Y=3時,以為條equation係IO3- + 3I- + 6H+ --> 2I2 + 3H2O。
其實你睇漏左d o野啦,euqation除左elements要balance, charge都要balance。如果Y = 3,條equation d charge左右唔balance,左面係-2而右面係0,所以唔得呀。明白嗎?
回應2
小白:
多謝你既提醒,我還是複習一下數學吧。因為之前一直都是溫化學物理(其他碰到沒碰)。多謝你肯熱心解答,謝謝。因為我一直都猶疑如果中六是選物理還是選化學好= =
其實中六的化學真的能夠解釋到我們不明白的嗎?即是有些問題老師總是說中六才會教,我怕到時又變成中七才會教,中七問時便是大學才會學。從小學到中學一直都是這樣,有極多問題都是不斷推遲說遲些教,不斷累加心中的疑問,真是非常辛苦。我都沒再對了,因為我本來化學成績就不好,只是臨考前溫了清了一點concept而已。所以能夠合格已經心足了。
化學友:
小白,化學友好明白你嘅疑問(講真,化學友讀F4時都有你嘅唸法),e d o野非三言兩語講得完。咁啦,你會考後有時間化學友再同你慢慢discuss o下啦。
回應3
kino_watashi:
1. 謝謝你的答覆雖然不是很明白你說甚麼但是我在yahoo!知識就找到了關於C-C這樣的一個回覆:True.formation of various (organic) compound is of due to the effective sharing of electron giving a stable covalent bond.(for your reference)the uniqueness of formation long carbon compound is called catenation.this is due to the effective sp3-sp3 sigma overlap of hybrids in C-C and C-H bond, i.e. relative high bond strength and is quite inert to ordinary reaction. therefore, nearly infinitely long or even stable hydrocarbons with cyclic carbon skeleton is formed. chain hydrocarbon is generalized by a formula CnH2n+2still, unsaturated carbon chain can be formed since neighboouring sp2 carbons contributes to effective p-p pi overlap making its formation gives extra stability.compared with silicon(also in group IV), covalent radius of Si-Si is larger. electrostatic attraction between nucleus and bonding electrons are less stronger than that in C-C bond. this is verified by the fact that in Si-Si, sigma overlap is less effective after hybridization. Si can only undergoes catenation up to 5 members
2.我想問下chem mc 42(3) 的amino acid formation 是不是out of syllabus 了?????
如果我chem 85%~90%對, mc 有45/50, 能不能有A?????
3. 我想問下, mc 47是C 而不是D 呢????因為我覺得only one variable 而其他都要the same, 所以我選了D.請指出我concept 上的錯誤, 謝謝~~
化學友:
1. kino_watashi,化學友覺得你chem嘅根底都好好。睇左你forward yahoo!知識段字,其實同化學友嘅explanation同一原理,不過因為e d係AL concept,所以未必睇得明。anyway o個ans係B o架啦。
2. 講真,kino_watashi e條有少少out c喇,因為我地CE多數學嘅monomer係有兩個一樣嘅functional groups,但其實兩個functional groups可以唔同,而都可以做到condensation polymer。e 條其實同CE 2005 條關於PLA 嘅long question一樣,都係有兩個唔同嘅functional groups。
如果你真係得到e o個分就一定A啦,恭喜你呀。
3. mc 47其實好難o架,會考後化學友先答你丫。 add oil la
回應4
Tom:
HIHI~我第一次來的。我對近日教的chem有一些不明,希望你們幫幫手。在simple Cu-Ag cell中,為什麼不可以用CuSO4做electrolyte而要用AgSO4??
化學友:
Tom,CuSO4都可以做electrolyte o架,唔一定要AgSO4。
o向chemical cell裡面electrolyte係幫助conduct electricity,basically任何electroyte(e.g. NaCl)都可以用o向chemical cell裡面o架。只不過唔同electroyte會o向通電後係Ag electrode出唔同嘅products。
例如,
用CuSO4做electrolyte,Cu就會deposite o向Ag electrode
用AgSO4做electrolyte,Ag就會deposite o向Ag electrode
用NaCl做electrolyte,hydrogen gas就會o向Ag electrode evolve
至於點解有唔同 嘅products出,就要用electrochemical series先可以解到。化學友唸你阿sir/miss遲d會教o架啦。
Tom,如果仲有唔明,就留言比化學友啦。
回應5
Eymon:
Dear Sir, one of my classmates said that in one year, only 92% or above correct can get an A in Chem, is it true? In fact, if I get 86% correct in 07 CE, to the best of your experience, do you think that I can get an A or not? Please tell me as soon as possible, since I am very worried and nervous.
化學友:
eymon, sorry呀,咁遲先答你。每一年CE chem mc大約45 out of 50就A,當然有時有例外啦,例如前兩年要o岩成47條先A。但係簡單o黎講每一年on average你mc岩45條long question有73-4分,overall就一定有A。如果你今年mc+long question平均有86%,咁就肯定有A o架啦(e排化學友聽到有d考生話今年分卷容易,要o岩好多先A。化學友肯定咁講一定唔係lor,其實好多考生都唔知自己錯左,以為白己o岩)。唔使worried呀eymon,比心機prepare好d a. maths呀,加油!!!
Schoolbag:
有條題我想問xIO3- + yI- + 6H+ ----->_I2 + _H2O
ANSWER:X=1 Y=5
我想知Y可吳可以系3?點解?
化學友:
schoolbag,化學友唸你當Y=3時,以為條equation係IO3- + 3I- + 6H+ --> 2I2 + 3H2O。
其實你睇漏左d o野啦,euqation除左elements要balance, charge都要balance。如果Y = 3,條equation d charge左右唔balance,左面係-2而右面係0,所以唔得呀。明白嗎?
回應2
小白:
多謝你既提醒,我還是複習一下數學吧。因為之前一直都是溫化學物理(其他碰到沒碰)。多謝你肯熱心解答,謝謝。因為我一直都猶疑如果中六是選物理還是選化學好= =
其實中六的化學真的能夠解釋到我們不明白的嗎?即是有些問題老師總是說中六才會教,我怕到時又變成中七才會教,中七問時便是大學才會學。從小學到中學一直都是這樣,有極多問題都是不斷推遲說遲些教,不斷累加心中的疑問,真是非常辛苦。我都沒再對了,因為我本來化學成績就不好,只是臨考前溫了清了一點concept而已。所以能夠合格已經心足了。
化學友:
小白,化學友好明白你嘅疑問(講真,化學友讀F4時都有你嘅唸法),e d o野非三言兩語講得完。咁啦,你會考後有時間化學友再同你慢慢discuss o下啦。
回應3
kino_watashi:
1. 謝謝你的答覆雖然不是很明白你說甚麼但是我在yahoo!知識就找到了關於C-C這樣的一個回覆:True.formation of various (organic) compound is of due to the effective sharing of electron giving a stable covalent bond.(for your reference)the uniqueness of formation long carbon compound is called catenation.this is due to the effective sp3-sp3 sigma overlap of hybrids in C-C and C-H bond, i.e. relative high bond strength and is quite inert to ordinary reaction. therefore, nearly infinitely long or even stable hydrocarbons with cyclic carbon skeleton is formed. chain hydrocarbon is generalized by a formula CnH2n+2still, unsaturated carbon chain can be formed since neighboouring sp2 carbons contributes to effective p-p pi overlap making its formation gives extra stability.compared with silicon(also in group IV), covalent radius of Si-Si is larger. electrostatic attraction between nucleus and bonding electrons are less stronger than that in C-C bond. this is verified by the fact that in Si-Si, sigma overlap is less effective after hybridization. Si can only undergoes catenation up to 5 members
2.我想問下chem mc 42(3) 的amino acid formation 是不是out of syllabus 了?????
如果我chem 85%~90%對, mc 有45/50, 能不能有A?????
3. 我想問下, mc 47是C 而不是D 呢????因為我覺得only one variable 而其他都要the same, 所以我選了D.請指出我concept 上的錯誤, 謝謝~~
化學友:
1. kino_watashi,化學友覺得你chem嘅根底都好好。睇左你forward yahoo!知識段字,其實同化學友嘅explanation同一原理,不過因為e d係AL concept,所以未必睇得明。anyway o個ans係B o架啦。
2. 講真,kino_watashi e條有少少out c喇,因為我地CE多數學嘅monomer係有兩個一樣嘅functional groups,但其實兩個functional groups可以唔同,而都可以做到condensation polymer。e 條其實同CE 2005 條關於PLA 嘅long question一樣,都係有兩個唔同嘅functional groups。
如果你真係得到e o個分就一定A啦,恭喜你呀。
3. mc 47其實好難o架,會考後化學友先答你丫。 add oil la
回應4
Tom:
HIHI~我第一次來的。我對近日教的chem有一些不明,希望你們幫幫手。在simple Cu-Ag cell中,為什麼不可以用CuSO4做electrolyte而要用AgSO4??
化學友:
Tom,CuSO4都可以做electrolyte o架,唔一定要AgSO4。
o向chemical cell裡面electrolyte係幫助conduct electricity,basically任何electroyte(e.g. NaCl)都可以用o向chemical cell裡面o架。只不過唔同electroyte會o向通電後係Ag electrode出唔同嘅products。
例如,
用CuSO4做electrolyte,Cu就會deposite o向Ag electrode
用AgSO4做electrolyte,Ag就會deposite o向Ag electrode
用NaCl做electrolyte,hydrogen gas就會o向Ag electrode evolve
至於點解有唔同 嘅products出,就要用electrochemical series先可以解到。化學友唸你阿sir/miss遲d會教o架啦。
Tom,如果仲有唔明,就留言比化學友啦。
回應5
Eymon:
Dear Sir, one of my classmates said that in one year, only 92% or above correct can get an A in Chem, is it true? In fact, if I get 86% correct in 07 CE, to the best of your experience, do you think that I can get an A or not? Please tell me as soon as possible, since I am very worried and nervous.
化學友:
eymon, sorry呀,咁遲先答你。每一年CE chem mc大約45 out of 50就A,當然有時有例外啦,例如前兩年要o岩成47條先A。但係簡單o黎講每一年on average你mc岩45條long question有73-4分,overall就一定有A。如果你今年mc+long question平均有86%,咁就肯定有A o架啦(e排化學友聽到有d考生話今年分卷容易,要o岩好多先A。化學友肯定咁講一定唔係lor,其實好多考生都唔知自己錯左,以為白己o岩)。唔使worried呀eymon,比心機prepare好d a. maths呀,加油!!!
2007年5月4日 星期五
點點回應
回應1
kino watashi:
Thanks~~
1. 我想問下, 為甚麼PVC 可以store concentrated sulphuric acid??? 聽說他們是有reaction 的.假設你mc 10 的答案對了, 為甚麼ethyl ethanoate 不可以store 在 expanded polystyrene container 呢?
2. mc 30 為甚麼第二條statement 不是第一條的正確解釋呢????
請賜教!!~~
化學友:
1. kino watashi,ethyl ethanoate係organic solvent,expanded polystyrene係會dissolve o向ethyl ethanoate。如果有機會,你可以試試將ethyl ethanoate加入expanded polystyrene cup,幾minutes後ethyl ethanoate變成白色,因為polystyrene dissolve左入去ethyl ethanoate到。
至於PVC係inert towards many chemicals例如conc. H2SO4。你可以睇睇2000 Paper II 38就明,PVC唔會同conc. H2SO4 react,佢只會soften at high temp。
2. Carbon can form a large number of compounds with long carbon chains嘅原因係因為C-C bond strong而唔係因為carbon atoms can share elctrons with one another。好多elements(例如Si)都可以share electrons with one another,但係就唔會form compounds with long Si chains因為Si-Si bond好弱。OK ma kino watashi?
回應2
Simon:
可唔可以解釋一下 07 chem mc 既第 5,26,27,39,42同第47題 ? 同埋 .. 我想問 .. mc 幾多分先肯定有C?萬分感激!!
化學友:
Simon,sorry ar,e排都係好忙,d mc遲一排再答你丫。
其實,如果有35條correct就一定有C o架啦,唔使太擔心。
回應3
wk:
你可否評論一下今年Alevel Chem 係深定淺?通常ALEVEL Chem 合格係幾多分?
化學友:
化學友覺得Paper I幾深呀,要考生對chem嘅concept好clear先答得到。Paper II就OK la,都應該可以得到一定分數。
wk,通常ALEVEL Chem有80分(out of 200分)就一定合格o架啦。
回應4
小白:
你好,我之前有問過你問題的,不知道你記不記得。之前上網對了是錯8題,對了你那個是錯了10題。可以解釋下那些問題的答案嗎?4,10*,21,30,31,33,36,38,41,51= ="另外,如果MC錯10題,長題目錯唔超過20分,有冇c = =?我連合格都唔肯定..錯好多....愈對先知愈多錯,一開始睇仲以為岩。
化學友:
化學友更係記得你啦。唔好意思呀,同Simon一樣化學友遲d再答你d mc丫。
小白,其實如果你真係得到e o個分就100%有C或以上,唔使驚呀。仲有呀小白,唔好再對啦,越對得多就會越發覺多錯o架啦,會影o向心情同埋跟住嘅subject嘅performance。相信化學友啦,你chem ok o架啦。
kino watashi:
Thanks~~
1. 我想問下, 為甚麼PVC 可以store concentrated sulphuric acid??? 聽說他們是有reaction 的.假設你mc 10 的答案對了, 為甚麼ethyl ethanoate 不可以store 在 expanded polystyrene container 呢?
2. mc 30 為甚麼第二條statement 不是第一條的正確解釋呢????
請賜教!!~~
化學友:
1. kino watashi,ethyl ethanoate係organic solvent,expanded polystyrene係會dissolve o向ethyl ethanoate。如果有機會,你可以試試將ethyl ethanoate加入expanded polystyrene cup,幾minutes後ethyl ethanoate變成白色,因為polystyrene dissolve左入去ethyl ethanoate到。
至於PVC係inert towards many chemicals例如conc. H2SO4。你可以睇睇2000 Paper II 38就明,PVC唔會同conc. H2SO4 react,佢只會soften at high temp。
2. Carbon can form a large number of compounds with long carbon chains嘅原因係因為C-C bond strong而唔係因為carbon atoms can share elctrons with one another。好多elements(例如Si)都可以share electrons with one another,但係就唔會form compounds with long Si chains因為Si-Si bond好弱。OK ma kino watashi?
回應2
Simon:
可唔可以解釋一下 07 chem mc 既第 5,26,27,39,42同第47題 ? 同埋 .. 我想問 .. mc 幾多分先肯定有C?萬分感激!!
化學友:
Simon,sorry ar,e排都係好忙,d mc遲一排再答你丫。
其實,如果有35條correct就一定有C o架啦,唔使太擔心。
回應3
wk:
你可否評論一下今年Alevel Chem 係深定淺?通常ALEVEL Chem 合格係幾多分?
化學友:
化學友覺得Paper I幾深呀,要考生對chem嘅concept好clear先答得到。Paper II就OK la,都應該可以得到一定分數。
wk,通常ALEVEL Chem有80分(out of 200分)就一定合格o架啦。
回應4
小白:
你好,我之前有問過你問題的,不知道你記不記得。之前上網對了是錯8題,對了你那個是錯了10題。可以解釋下那些問題的答案嗎?4,10*,21,30,31,33,36,38,41,51= ="另外,如果MC錯10題,長題目錯唔超過20分,有冇c = =?我連合格都唔肯定..錯好多....愈對先知愈多錯,一開始睇仲以為岩。
化學友:
化學友更係記得你啦。唔好意思呀,同Simon一樣化學友遲d再答你d mc丫。
小白,其實如果你真係得到e o個分就100%有C或以上,唔使驚呀。仲有呀小白,唔好再對啦,越對得多就會越發覺多錯o架啦,會影o向心情同埋跟住嘅subject嘅performance。相信化學友啦,你chem ok o架啦。
2007年5月2日 星期三
2007 CE mc
同學仔,今日考得好唔好呀?化學友覺得今年份卷都唔算容易,especially mc潻呀,對考生chem嘅要求都幾高,好易會做錯。化學友都有d students失手啦。剛剛見到有 discussion forum post左d mc answer出o黎,但係幾個forum嘅answer都有d錯。化學友決定post d correct ans 比你地睇睇:
1-10:
BDDBDDABDD
11-20:
ADACCBCBAA
21-30:
ACCBDBDCCB
31-40:
AABCDDADAA
41-50:
BDACDBCCBC
anyway,同學仔今日嘅chem已經過去啦,考得好唔好都要比心機prepare Eng同埋Mathso架啦,you should concentrate on your coming exam。(祝聽日要考econ嘅同學有好成績啦)
回應
chemson:
化學友覺得今年LQ,MC幾分先A到?難度怎樣?
化學友:
chemson,今年份卷都幾難。因為要拉curve,所以要改完先知幾多分有A。以住就大約mc 45條(out of 50條) long question 73-4(out of 90分)就A。不過,化學友覺得今年會低d o架啦。
1-10:
BDDBDDABDD
11-20:
ADACCBCBAA
21-30:
ACCBDBDCCB
31-40:
AABCDDADAA
41-50:
BDACDBCCBC
anyway,同學仔今日嘅chem已經過去啦,考得好唔好都要比心機prepare Eng同埋Mathso架啦,you should concentrate on your coming exam。(祝聽日要考econ嘅同學有好成績啦)
回應
chemson:
化學友覺得今年LQ,MC幾分先A到?難度怎樣?
化學友:
chemson,今年份卷都幾難。因為要拉curve,所以要改完先知幾多分有A。以住就大約mc 45條(out of 50條) long question 73-4(out of 90分)就A。不過,化學友覺得今年會低d o架啦。
2007年5月1日 星期二
回應(會考化學)
回應1
maryuen:
我想知道tin要比copper reactive ma?
化學友:
係呀Maryuen,tin係比copper reactive。
Reactivity series: Fe>Sn>Pb>Cu
回應2
ii:
請問chem ge past paper,98-04年ge mc 同paper1 有邊幾題係out c?thank you
化學友:
98 Paper I Q.3(a), 4, 6(a)(ii)(2)
99 Paper I Q. 7(b)(iv), Q8(b)(i),(iii)
2000 PaperI Q.5, Q.9(c)(ii),(d)
2001 Paper I Q.3(b), Q.8(b)
2002 Paper I Q.1(a), Q.3(a)
2003 Paper I Q.6(b)
2004 Paper I Q.9(c)(i)
98 Paper II 8, 35, 37, 50
99 Paper II 14, 23, 47, 49, 50
2000 Paper II 10, 26
2001 Paper II 13, 39, 48
2002 Paper II 4, 15, 25, 42, 50
2003 Paper II 4, 34, 47
2004Paper II 5, 19, 35, 50
回應3
Chemson:
1. 不好意思,還有一條問題06 LQ 最後果題,determine molar volume marking scheme話用excess HCl我答左excess Mg ribbon有冇分?
2. 我在某本ex.見到條問題,叫我寫equation+observation,chlroine water+iodine solution(i.e.iodine dissolve in potassium iodine)iodine dissolve in potassium iodine即係點?有I2(aq)定I-(aq)?還是兩個都有呢?
化學友:
1. 因為如果用咗excess Mg ribbon,係難d睇得出HCl係唔係完全用晒,因為任何時間都見到有Mg剩,所以呢個寫法唔係幾好,不過都有分。
2. chemson記住I2 dissolved in KI = I2(aq)。因為I2唔係好溶於水,要用potassium iodine增加佢嘅solubility(至於explanation係out c)。
回應4
虫:
除左CaCO3外燒CO3 2- compound既野會唔會出 CO2??
化學友:
會呀,所有metal carbonate都會 give CO2 under heating除左K2CO3同埋Na2CO3。
回應5
Kit:
你好: 我想請問 thermal decomposition的問題,有什麼carbonate salt,nitrate salt,sulphate salt will decompose on heating?Thank you very much!
化學友:
化學友已經係2007年4月12日 星期四嘅回應整咗個表俾同學參考喇,你去see see la。
回應6
Kwok:
1. is heat is needed for the production of NH3 from NH4+ + OH-?
2. 1991年mc第36題why substitution is considered as violent?
化學友:
1. 要呀,會考一定要heat或者warm。
2. 因為呢個reaction係有光情況下都幾勁,好快完成,所以都算violent。
maryuen:
我想知道tin要比copper reactive ma?
化學友:
係呀Maryuen,tin係比copper reactive。
Reactivity series: Fe>Sn>Pb>Cu
回應2
ii:
請問chem ge past paper,98-04年ge mc 同paper1 有邊幾題係out c?thank you
化學友:
98 Paper I Q.3(a), 4, 6(a)(ii)(2)
99 Paper I Q. 7(b)(iv), Q8(b)(i),(iii)
2000 PaperI Q.5, Q.9(c)(ii),(d)
2001 Paper I Q.3(b), Q.8(b)
2002 Paper I Q.1(a), Q.3(a)
2003 Paper I Q.6(b)
2004 Paper I Q.9(c)(i)
98 Paper II 8, 35, 37, 50
99 Paper II 14, 23, 47, 49, 50
2000 Paper II 10, 26
2001 Paper II 13, 39, 48
2002 Paper II 4, 15, 25, 42, 50
2003 Paper II 4, 34, 47
2004Paper II 5, 19, 35, 50
回應3
Chemson:
1. 不好意思,還有一條問題06 LQ 最後果題,determine molar volume marking scheme話用excess HCl我答左excess Mg ribbon有冇分?
2. 我在某本ex.見到條問題,叫我寫equation+observation,chlroine water+iodine solution(i.e.iodine dissolve in potassium iodine)iodine dissolve in potassium iodine即係點?有I2(aq)定I-(aq)?還是兩個都有呢?
化學友:
1. 因為如果用咗excess Mg ribbon,係難d睇得出HCl係唔係完全用晒,因為任何時間都見到有Mg剩,所以呢個寫法唔係幾好,不過都有分。
2. chemson記住I2 dissolved in KI = I2(aq)。因為I2唔係好溶於水,要用potassium iodine增加佢嘅solubility(至於explanation係out c)。
回應4
虫:
除左CaCO3外燒CO3 2- compound既野會唔會出 CO2??
化學友:
會呀,所有metal carbonate都會 give CO2 under heating除左K2CO3同埋Na2CO3。
回應5
Kit:
你好: 我想請問 thermal decomposition的問題,有什麼carbonate salt,nitrate salt,sulphate salt will decompose on heating?Thank you very much!
化學友:
化學友已經係2007年4月12日 星期四嘅回應整咗個表俾同學參考喇,你去see see la。
回應6
Kwok:
1. is heat is needed for the production of NH3 from NH4+ + OH-?
2. 1991年mc第36題why substitution is considered as violent?
化學友:
1. 要呀,會考一定要heat或者warm。
2. 因為呢個reaction係有光情況下都幾勁,好快完成,所以都算violent。
會考化學(14)
o向係第八章塑膠與清潔劑裡面,同學留意以下幾點。

2. 各種treat plastic wastes(處理塑膠棄物) method (incineration焚化、landfill堆 田同埋pyrolysis熱解)嘅優、缺點 。可以參考以下嘅pastpaper:
1. 首先要記住塑膠嘅分類,總共有兩種:

可以參考以下嘅pastpaper:
2002 Paper I 8c
2005 Paper I 5
2006 Paper II 43
2006 Paper I 11
2. 各種treat plastic wastes(處理塑膠棄物) method (incineration焚化、landfill堆 田同埋pyrolysis熱解)嘅優、缺點 。可以參考以下嘅pastpaper:
1996 Paper I 7b
2003 Paper I 5
2003 Paper I 5
2004 Paper I 6c
(iii) Soap(肥皂)係由fat或者oil同埋NaOH製成(以前講過),Soapless detergent (非 皂性清潔劑)係由petroleum(石油)同sulphuric acid(硫酸) and sodium hydroxide化合而成 。可以參考以下嘅pastpaper:
1995 Paper I 9a
2001 Paper I 6a
2005 Paper II 32
2006 Paper II 42
會考化學(13)
o向七章有機化學(organic chemistry)裡面,同學仔要留意以下幾點。
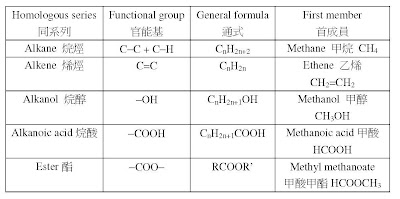
2. 要溫熟cracking process(裂解過程)、reaction condition(反應條件)、equation(方 程式)同埋e個reaction advantages(反應嘅優點) 。睇睇下面嘅pastpaper:
3. 記住各種pollutant(污染物)嘅cause(成因)、reducing method(減少方法)同埋 effect on human and environment(對人同環境影響) (注意呀increasing height of chimney (增加煙囪高度)係唔可以減少空氣污染)。 睇睇下面嘅pastpaper:
4. 注意alkanoic acid(烷酸)同埋ester(酯)嘅關係,它們只係有相同嘅molecular formula(分子式),佢哋係唔同類型嘅compound,有唔同嘅reaction同埋 physical properties(物理性質),例如 b.p.

1. 首先要記住e課提及過嘅唔同種類嘅organic compounds(有機化合物):

睇睇下面嘅pastpaper:
2001 Paper II 32
2005 Paper II 21
2. 要溫熟cracking process(裂解過程)、reaction condition(反應條件)、equation(方 程式)同埋e個reaction advantages(反應嘅優點) 。睇睇下面嘅pastpaper:
1999 Paper I 9b
2003 Paper I 7b
2003 Paper I 7b
2005 Paper II 2
3. 記住各種pollutant(污染物)嘅cause(成因)、reducing method(減少方法)同埋 effect on human and environment(對人同環境影響) (注意呀increasing height of chimney (增加煙囪高度)係唔可以減少空氣污染)。 睇睇下面嘅pastpaper:
1999 Paper I 3
1999 Paper II 30
1999 Paper II 30
2001 Paper II 14
2003 Paper I 1b
4. 注意alkanoic acid(烷酸)同埋ester(酯)嘅關係,它們只係有相同嘅molecular formula(分子式),佢哋係唔同類型嘅compound,有唔同嘅reaction同埋 physical properties(物理性質),例如 b.p.

睇睇下面嘅pastpaper:
2000 Paper II 13
2003 Paper II 38
訂閱:
意見 (Atom)





