回應1
maryuen:
我想知道tin要比copper reactive ma?
化學友:
係呀Maryuen,tin係比copper reactive。
Reactivity series: Fe>Sn>Pb>Cu
回應2
ii:
請問chem ge past paper,98-04年ge mc 同paper1 有邊幾題係out c?thank you
化學友:
98 Paper I Q.3(a), 4, 6(a)(ii)(2)
99 Paper I Q. 7(b)(iv), Q8(b)(i),(iii)
2000 PaperI Q.5, Q.9(c)(ii),(d)
2001 Paper I Q.3(b), Q.8(b)
2002 Paper I Q.1(a), Q.3(a)
2003 Paper I Q.6(b)
2004 Paper I Q.9(c)(i)
98 Paper II 8, 35, 37, 50
99 Paper II 14, 23, 47, 49, 50
2000 Paper II 10, 26
2001 Paper II 13, 39, 48
2002 Paper II 4, 15, 25, 42, 50
2003 Paper II 4, 34, 47
2004Paper II 5, 19, 35, 50
回應3
Chemson:
1. 不好意思,還有一條問題06 LQ 最後果題,determine molar volume marking scheme話用excess HCl我答左excess Mg ribbon有冇分?
2. 我在某本ex.見到條問題,叫我寫equation+observation,chlroine water+iodine solution(i.e.iodine dissolve in potassium iodine)iodine dissolve in potassium iodine即係點?有I2(aq)定I-(aq)?還是兩個都有呢?
化學友:
1. 因為如果用咗excess Mg ribbon,係難d睇得出HCl係唔係完全用晒,因為任何時間都見到有Mg剩,所以呢個寫法唔係幾好,不過都有分。
2. chemson記住I2 dissolved in KI = I2(aq)。因為I2唔係好溶於水,要用potassium iodine增加佢嘅solubility(至於explanation係out c)。
回應4
虫:
除左CaCO3外燒CO3 2- compound既野會唔會出 CO2??
化學友:
會呀,所有metal carbonate都會 give CO2 under heating除左K2CO3同埋Na2CO3。
回應5
Kit:
你好: 我想請問 thermal decomposition的問題,有什麼carbonate salt,nitrate salt,sulphate salt will decompose on heating?Thank you very much!
化學友:
化學友已經係2007年4月12日 星期四嘅回應整咗個表俾同學參考喇,你去see see la。
回應6
Kwok:
1. is heat is needed for the production of NH3 from NH4+ + OH-?
2. 1991年mc第36題why substitution is considered as violent?
化學友:
1. 要呀,會考一定要heat或者warm。
2. 因為呢個reaction係有光情況下都幾勁,好快完成,所以都算violent。
2007年5月1日 星期二
會考化學(14)
o向係第八章塑膠與清潔劑裡面,同學留意以下幾點。

2. 各種treat plastic wastes(處理塑膠棄物) method (incineration焚化、landfill堆 田同埋pyrolysis熱解)嘅優、缺點 。可以參考以下嘅pastpaper:
1. 首先要記住塑膠嘅分類,總共有兩種:

可以參考以下嘅pastpaper:
2002 Paper I 8c
2005 Paper I 5
2006 Paper II 43
2006 Paper I 11
2. 各種treat plastic wastes(處理塑膠棄物) method (incineration焚化、landfill堆 田同埋pyrolysis熱解)嘅優、缺點 。可以參考以下嘅pastpaper:
1996 Paper I 7b
2003 Paper I 5
2003 Paper I 5
2004 Paper I 6c
(iii) Soap(肥皂)係由fat或者oil同埋NaOH製成(以前講過),Soapless detergent (非 皂性清潔劑)係由petroleum(石油)同sulphuric acid(硫酸) and sodium hydroxide化合而成 。可以參考以下嘅pastpaper:
1995 Paper I 9a
2001 Paper I 6a
2005 Paper II 32
2006 Paper II 42
會考化學(13)
o向七章有機化學(organic chemistry)裡面,同學仔要留意以下幾點。
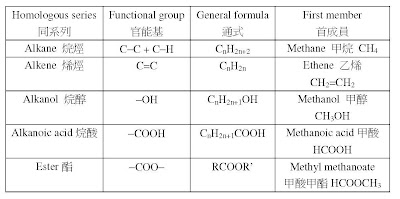
2. 要溫熟cracking process(裂解過程)、reaction condition(反應條件)、equation(方 程式)同埋e個reaction advantages(反應嘅優點) 。睇睇下面嘅pastpaper:
3. 記住各種pollutant(污染物)嘅cause(成因)、reducing method(減少方法)同埋 effect on human and environment(對人同環境影響) (注意呀increasing height of chimney (增加煙囪高度)係唔可以減少空氣污染)。 睇睇下面嘅pastpaper:
4. 注意alkanoic acid(烷酸)同埋ester(酯)嘅關係,它們只係有相同嘅molecular formula(分子式),佢哋係唔同類型嘅compound,有唔同嘅reaction同埋 physical properties(物理性質),例如 b.p.

1. 首先要記住e課提及過嘅唔同種類嘅organic compounds(有機化合物):

睇睇下面嘅pastpaper:
2001 Paper II 32
2005 Paper II 21
2. 要溫熟cracking process(裂解過程)、reaction condition(反應條件)、equation(方 程式)同埋e個reaction advantages(反應嘅優點) 。睇睇下面嘅pastpaper:
1999 Paper I 9b
2003 Paper I 7b
2003 Paper I 7b
2005 Paper II 2
3. 記住各種pollutant(污染物)嘅cause(成因)、reducing method(減少方法)同埋 effect on human and environment(對人同環境影響) (注意呀increasing height of chimney (增加煙囪高度)係唔可以減少空氣污染)。 睇睇下面嘅pastpaper:
1999 Paper I 3
1999 Paper II 30
1999 Paper II 30
2001 Paper II 14
2003 Paper I 1b
4. 注意alkanoic acid(烷酸)同埋ester(酯)嘅關係,它們只係有相同嘅molecular formula(分子式),佢哋係唔同類型嘅compound,有唔同嘅reaction同埋 physical properties(物理性質),例如 b.p.

睇睇下面嘅pastpaper:
2000 Paper II 13
2003 Paper II 38
2007年4月30日 星期一
回應(會考化學)
回應1
pele159:
1. 請問 HCl(aq) & HCl(g)係咪compound ?
2. 地球最多的 element 係咪 oxygen ??地球最多的 metal咪 Iron ??
3. conc.H2SO4 + heat 係咪react cu ?Iron heat conc.H2SO4 係會出咩呀 ??
4. 係咪只有HNO3 stored in brown bottles ?
5. 其實而家應該做出面練習的mock 定係 redo 錯嘅野,溫下書好呀 ??
化學友:
1. HCl係covalent compound, (aq)同埋(g)只係代表唔同嘅states。
2. 地球最多嘅 element 係oxygen(之前寫錯,多謝網友指正)。地球最多嘅 metal係 aluminium。
3. Cu + 2H2SO4 --> CuSO4 + SO2 + 2H2O
Iron heat conc.H2SO4:
Fe + 2H2SO4 --> FeSO4 + SO2 + 2H2O
4. 除咗HNO3, 仲有AgNO3同埋KMnO4。CE chem大約知咁多就得o架喇
5. 化學友覺得你應該redo錯嘅野喇,同埋溫番dnote,唔好剩係做題目,會更有效。
回應2
pele159:
1. 86 Q.2The melting points of diamond, sodium chloride and potassium increase in the order:
A. diamond, sodium chloride, potassium
B. potassium, sodium chloride, diamond
C. sodium chloride, diamond, potassium
D. sodium chloride, potassium, diamond
請問 Metallic bond 和 ionic bond , 邊果melting points 大 d 呀 ?
2. 86 Q.38Bromine exists in two isotopic forms, 3579Br and 3581Br . The relative atomic mass of bromine is79.9. Which of the following statements is/are correct?
(1) The relative abundance of each isotopic form is about the same.
(2) The two isotopes have different numbers of protons.
(3) The two isotopes have different numbers of neutrons.
A. (2) only
B. (3) only
C. (1) and (2) only
D. (1) and (3) only
請問(1)點解arm 呀 ?
3. 87 Q.32(1) Nitrogen does not react readily with other elements or compounds.
(2) The outermost electron shell of the nitrogen atom is completely filled.
點解(1) 係arm 呀 ?? 乜Nitrogen 唔係covalent bond ?? 如果要does not react readily with other , 乜唔係 Noble gas 果d咩 ??
化學友:
1. 一般o黎講ionic bond強過metallic bond。所以ionic compound 嘅melting point 係高過metal。
2. 其實當年marking係錯o架。因為79Br有55%而81Br有45%,所以relative abundance is not about the same。
3. nitrogen(N2)內有nitrogen-nitrogen triple bond。化學反應時,需要好高energy才可以break nitrogen-nitrogen triple bond,所以佢係好少reaction,好unreactive。但係佢都有reaction嘅,例如: 3Mg + N2 à Mg3N2。
當然noble gases比N2 unreactive得多啦。
回應3
carmenlee:
你好,我有個chem既問題想問下OCl-會turn red litmus paper blue then white啦;咁H2SO3 turn完blue litmus paper red之後會唔會turn white?p.s.我見d solution都冇寫佢會turn white,但係SO3 2-唔係都bleach到野架咩?
化學友:
carmenlee,你係correct o架。但係SO32-嘅bleaching power比較mild,要long time先可以turn litmus paper white,所以ans只係寫即時observation。
回應4
sinying:
我想問下呢:alkonal加Na,CH3COOH加Na係唔係會都出H2??我睇書話佢地都係會出OH- ion,所以有H2放但咩唔係只係H+ion加Na先會出H2咩?OH-加Na為何出H2,而且CH3COOH唔係ACID來的嗎?咩唔係會出H+...........THX
化學友:
係呀,佢地會出H2 o架:
2CH3CH2OH + 2Na --> 2CH3CH2ONa + H2
2CH3COOH + 2Na --> 2CH3COONa + H2
sinying,化學友唸你睇錯o野喇。凡親一隻compound有H+或者hydroxyl group(-OH group 唔係OH- ion)就可以同Na react to give H2 o架啦。CH3CH2OH有 -OH group而CH3COOH有H+,所以有reaction。
pele159:
1. 請問 HCl(aq) & HCl(g)係咪compound ?
2. 地球最多的 element 係咪 oxygen ??地球最多的 metal咪 Iron ??
3. conc.H2SO4 + heat 係咪react cu ?Iron heat conc.H2SO4 係會出咩呀 ??
4. 係咪只有HNO3 stored in brown bottles ?
5. 其實而家應該做出面練習的mock 定係 redo 錯嘅野,溫下書好呀 ??
化學友:
1. HCl係covalent compound, (aq)同埋(g)只係代表唔同嘅states。
2. 地球最多嘅 element 係oxygen(之前寫錯,多謝網友指正)。地球最多嘅 metal係 aluminium。
3. Cu + 2H2SO4 --> CuSO4 + SO2 + 2H2O
Iron heat conc.H2SO4:
Fe + 2H2SO4 --> FeSO4 + SO2 + 2H2O
4. 除咗HNO3, 仲有AgNO3同埋KMnO4。CE chem大約知咁多就得o架喇
5. 化學友覺得你應該redo錯嘅野喇,同埋溫番dnote,唔好剩係做題目,會更有效。
回應2
pele159:
1. 86 Q.2The melting points of diamond, sodium chloride and potassium increase in the order:
A. diamond, sodium chloride, potassium
B. potassium, sodium chloride, diamond
C. sodium chloride, diamond, potassium
D. sodium chloride, potassium, diamond
請問 Metallic bond 和 ionic bond , 邊果melting points 大 d 呀 ?
2. 86 Q.38Bromine exists in two isotopic forms, 3579Br and 3581Br . The relative atomic mass of bromine is79.9. Which of the following statements is/are correct?
(1) The relative abundance of each isotopic form is about the same.
(2) The two isotopes have different numbers of protons.
(3) The two isotopes have different numbers of neutrons.
A. (2) only
B. (3) only
C. (1) and (2) only
D. (1) and (3) only
請問(1)點解arm 呀 ?
3. 87 Q.32(1) Nitrogen does not react readily with other elements or compounds.
(2) The outermost electron shell of the nitrogen atom is completely filled.
點解(1) 係arm 呀 ?? 乜Nitrogen 唔係covalent bond ?? 如果要does not react readily with other , 乜唔係 Noble gas 果d咩 ??
化學友:
1. 一般o黎講ionic bond強過metallic bond。所以ionic compound 嘅melting point 係高過metal。
2. 其實當年marking係錯o架。因為79Br有55%而81Br有45%,所以relative abundance is not about the same。
3. nitrogen(N2)內有nitrogen-nitrogen triple bond。化學反應時,需要好高energy才可以break nitrogen-nitrogen triple bond,所以佢係好少reaction,好unreactive。但係佢都有reaction嘅,例如: 3Mg + N2 à Mg3N2。
當然noble gases比N2 unreactive得多啦。
回應3
carmenlee:
你好,我有個chem既問題想問下OCl-會turn red litmus paper blue then white啦;咁H2SO3 turn完blue litmus paper red之後會唔會turn white?p.s.我見d solution都冇寫佢會turn white,但係SO3 2-唔係都bleach到野架咩?
化學友:
carmenlee,你係correct o架。但係SO32-嘅bleaching power比較mild,要long time先可以turn litmus paper white,所以ans只係寫即時observation。
回應4
sinying:
我想問下呢:alkonal加Na,CH3COOH加Na係唔係會都出H2??我睇書話佢地都係會出OH- ion,所以有H2放但咩唔係只係H+ion加Na先會出H2咩?OH-加Na為何出H2,而且CH3COOH唔係ACID來的嗎?咩唔係會出H+...........THX
化學友:
係呀,佢地會出H2 o架:
2CH3CH2OH + 2Na --> 2CH3CH2ONa + H2
2CH3COOH + 2Na --> 2CH3COONa + H2
sinying,化學友唸你睇錯o野喇。凡親一隻compound有H+或者hydroxyl group(-OH group 唔係OH- ion)就可以同Na react to give H2 o架啦。CH3CH2OH有 -OH group而CH3COOH有H+,所以有reaction。
2007年4月29日 星期日
會考化學(12)
係第五章Redox(氧化還原)裡面,同學仔記住要留意以下幾點:
1. 連接兩個half-cells(半電池)之間所用嘅salt bridge(鹽橋),目的嘅係容許half-cells內嘅ions(離子)互相通過。 睇睇下面嘅pastpaer:
1995 Paper I 9b
2004 Paper II 40
2006 Paper II 40
2. 好多時卷二都有條計氧化數同埋判斷邊條係氧化還原反應嘅mc,e兩年好多考生都錯,所以要小心。睇睇下面嘅pastpaer:
2005 Paper II 8
2006 Paper II 3
2006 Paper II 27
3. 如果我地想acidify potassium permanganate(酸化高錳酸鉀)或者係sodium dichromate(重鉻酸鈉) 所用嘅acid應該係dil. sulphuric acid (硫酸)。唔用HCl(aq)(氫氯酸) 嘅原因係MnO4-會oxidize(氧化)Cl-(氯離子)。 唔用HNO3(aq)(硝酸)嘅原因係HNO3自已都係1隻oxidizing agent(氧化劑),佢嘅存在會同MnO4-或Cr2O72-爭住oxidize另一隻reducing agent,出現unexpected result。睇睇下面嘅pastpaer:
1999 Paper II 15
4. 比較Fe2+同埋Fe3+嘅difference:
Fe2+
colour: pale green(淺綠色)
+ NaOH: dirty green precipitate
+acidified KMnO4: pale green --> yellow
Fe3+
colour: yellow(黃色)
+NaOH: brown precipitate
+acidified KMnO4: no observable change
5. 解釋reaction係唔係redox, 只需寫出reducing agent(還原劑) 或者係oxidizing agent (氧化劑) 嘅 change of oxidation number (氧化數轉變)。例如,2Na + Cl2 --> 2NaCl係redox,因為嘅O.N. of Na increases from 0 to +1 / O.N. of Cl decreases from 0 to -1。
6. 注意在electroplating(電鍍)裡,anode(陽極)、cathode(陰極)和electrolyte(電解質)嘅配搭。睇睇下面嘅pastpaer:
1993 Paper I 2a
1995 Paper II 11
1996 Paper I 9b
係第六章重要嘅工業產品裡面,同學仔要留意以下幾點:
1. Chlorine(氯氣)溶係NaOH係一個redox reaction(氧化還原反應),而最重要係Cl同一時間 oxidized同埋reduced。
Cl2 + 2NaOH --> NaCl + NaOCl + H2O
Cl嘅O.N.changes from 0 to -1(Cl2--> NaCl)
at the same time Cl嘅O.N. changes from 0 to +1(Cl2 --> NaOCl)
睇睇下面嘅pastpaer:
1997 Paper II 21
1999 年 Paper II 36
2. compare chlorite ion(次氯酸根離子)同埋sulphite ion(亞硫酸根離子)作為bleachingagent(漂白劑)嘅分別:

睇睇下面嘅pastpaer:
1998 Paper II 32
2001 Paper I 9d
2005 Paper II 17
3. Conc. sulphuric acid(濃硫酸)同dilute sulphuric acid(稀硫酸)不同嘅特性。只有conc. sulphuric acid有 oxidzing property同埋dehydrating property,而di. sulphuric acid就冇。
Oxidizing(氧化性) of conc. sulphuric acid:
Cu + 2H2SO4 --> CuSO4 + SO2 + H2O
C + 2H2SO4 --> CO2 + SO2 + 2H2O
S + 2H2SO4 --> 3SO2 + 2H2O
Dehydrating(脫水性) of conc. sulphuric acid:
CuSO4.5H2O --> CuSO4 + 5H2O
C12H22O11 --> 12C + 11H2O
(C6H10O5)n --> 6nC + 5nH2O
4. conc. sulphuric acid同埋dil. sulphuric acid都有嘅相同特性。
同alkali solution(鹼溶液) react:
H2SO4 + 2NaOH -->Na2SO4 + 2H2O
同carbonate salt(碳酸鹽)嘅反應:
H2SO4 + Na2CO3 --> Na2SO4 + CO2 + H2O
1999 Paper II 7
2001 Paper I 9c
5. o向計算molar volume(摩爾體積)時,要留意以下兩點
i. 在same no. of moles(相同的摩爾)下,gas嘅體積比liquid同埋solid大好多,所以記住一mole N2同一moleNaCl或者一moleH2O嘅volume係唔同。睇睇下面嘅pastpaer:
1998 Paper II 46
2000 Paper II 48
ii. 留意計diatomic gas molecule(雙原子氣體分子)時,係問atom(原子) 定係問molecule(分子),d考生成日都wrong o架。睇睇下面嘅pastpaer:
2001 Paper II 27
1. 連接兩個half-cells(半電池)之間所用嘅salt bridge(鹽橋),目的嘅係容許half-cells內嘅ions(離子)互相通過。 睇睇下面嘅pastpaer:
1995 Paper I 9b
2004 Paper II 40
2006 Paper II 40
2. 好多時卷二都有條計氧化數同埋判斷邊條係氧化還原反應嘅mc,e兩年好多考生都錯,所以要小心。睇睇下面嘅pastpaer:
2005 Paper II 8
2006 Paper II 3
2006 Paper II 27
3. 如果我地想acidify potassium permanganate(酸化高錳酸鉀)或者係sodium dichromate(重鉻酸鈉) 所用嘅acid應該係dil. sulphuric acid (硫酸)。唔用HCl(aq)(氫氯酸) 嘅原因係MnO4-會oxidize(氧化)Cl-(氯離子)。 唔用HNO3(aq)(硝酸)嘅原因係HNO3自已都係1隻oxidizing agent(氧化劑),佢嘅存在會同MnO4-或Cr2O72-爭住oxidize另一隻reducing agent,出現unexpected result。睇睇下面嘅pastpaer:
1999 Paper II 15
4. 比較Fe2+同埋Fe3+嘅difference:
Fe2+
colour: pale green(淺綠色)
+ NaOH: dirty green precipitate
+acidified KMnO4: pale green --> yellow
Fe3+
colour: yellow(黃色)
+NaOH: brown precipitate
+acidified KMnO4: no observable change
5. 解釋reaction係唔係redox, 只需寫出reducing agent(還原劑) 或者係oxidizing agent (氧化劑) 嘅 change of oxidation number (氧化數轉變)。例如,2Na + Cl2 --> 2NaCl係redox,因為嘅O.N. of Na increases from 0 to +1 / O.N. of Cl decreases from 0 to -1。
6. 注意在electroplating(電鍍)裡,anode(陽極)、cathode(陰極)和electrolyte(電解質)嘅配搭。睇睇下面嘅pastpaer:
1993 Paper I 2a
1995 Paper II 11
1996 Paper I 9b
係第六章重要嘅工業產品裡面,同學仔要留意以下幾點:
1. Chlorine(氯氣)溶係NaOH係一個redox reaction(氧化還原反應),而最重要係Cl同一時間 oxidized同埋reduced。
Cl2 + 2NaOH --> NaCl + NaOCl + H2O
Cl嘅O.N.changes from 0 to -1(Cl2--> NaCl)
at the same time Cl嘅O.N. changes from 0 to +1(Cl2 --> NaOCl)
睇睇下面嘅pastpaer:
1997 Paper II 21
1999 年 Paper II 36
2. compare chlorite ion(次氯酸根離子)同埋sulphite ion(亞硫酸根離子)作為bleachingagent(漂白劑)嘅分別:

睇睇下面嘅pastpaer:
1998 Paper II 32
2001 Paper I 9d
2005 Paper II 17
3. Conc. sulphuric acid(濃硫酸)同dilute sulphuric acid(稀硫酸)不同嘅特性。只有conc. sulphuric acid有 oxidzing property同埋dehydrating property,而di. sulphuric acid就冇。
Oxidizing(氧化性) of conc. sulphuric acid:
Cu + 2H2SO4 --> CuSO4 + SO2 + H2O
C + 2H2SO4 --> CO2 + SO2 + 2H2O
S + 2H2SO4 --> 3SO2 + 2H2O
Dehydrating(脫水性) of conc. sulphuric acid:
CuSO4.5H2O --> CuSO4 + 5H2O
C12H22O11 --> 12C + 11H2O
(C6H10O5)n --> 6nC + 5nH2O
4. conc. sulphuric acid同埋dil. sulphuric acid都有嘅相同特性。
同alkali solution(鹼溶液) react:
H2SO4 + 2NaOH -->Na2SO4 + 2H2O
同carbonate salt(碳酸鹽)嘅反應:
H2SO4 + Na2CO3 --> Na2SO4 + CO2 + H2O
睇睇下面嘅pastpaer:
1998 Paper I 8a1999 Paper II 7
2001 Paper I 9c
5. o向計算molar volume(摩爾體積)時,要留意以下兩點
i. 在same no. of moles(相同的摩爾)下,gas嘅體積比liquid同埋solid大好多,所以記住一mole N2同一moleNaCl或者一moleH2O嘅volume係唔同。睇睇下面嘅pastpaer:
1998 Paper II 46
2000 Paper II 48
ii. 留意計diatomic gas molecule(雙原子氣體分子)時,係問atom(原子) 定係問molecule(分子),d考生成日都wrong o架。睇睇下面嘅pastpaer:
2001 Paper II 27
訂閱:
文章 (Atom)





