2007年6月10日 星期日
回來了
無名人:
老師,我想問問在electrolysis 裏用dilute sodium chloride 所得的product 是否和concentrated 的相同(chlorine 和 hydrogen)?我校老師這樣的分:very dilute, dilute, concentrated where dilute=concentrated。
化學友:
無名人叫我做化學友得啦,唔好叫老師喇。你學校o既老師係o岩o架。雖然真實上both oxygen and chlorine may be evloved at the anode at the same time當 electrolyzing dilute NaCl,但係o向CE level為左令各同學仔容易d讀,就simplify左當dilute and concentrated NaCl都只係出Cl2。仲有呀,正常o黎講CE只係會考electrolyzing very dilute NaCl (出O2)同埋electrolyzing concentrated NaCl (出Cl2),好少好少出electrotrlyzing dilute NaCl o架。 OK ma無名人?
natalie chemistry:
Hello~化學友...第一次上黎問你野...好多呀sir都話08年既chemistry會好難咁你又覺得點呢??^^因為我係08年會考生...所以都幾擔心呢個問題thx
化學友:
natalie化學友唸d阿sir話08年深係冇乜根據o架,因為好多時份卷深唔深係depend on o個年考評局examination board d members出卷o既style適唔適合o個年d考生答,通常深唔深都係事後孔明。好似CE chem咁,2002 - 2005都唔係好深,2006就勁深,2007又少少深(雖然好多人話今年份卷容易,但係其實好多考生section B都答得好差。),其實冇乜pattern可見。
仲有呀,化學友發覺無論o個年深定淺,化學友大約都係有差唔多數目o既學生欏A-C,因為CE chem係拉curve。如果個考生係能力高 o既,無論份卷幾深佢o既成績都係排得好前,最後拉curve後佢都係會欏A。所以natalie信化學友丫,只要妳e家開始盡力提升妳d chem o既ability,無論2008年份卷幾深,妳o既成績就o吾會差得去邊o架啦。add oil呀!
送首歌比各位同學仔呀,e首歌近排比左好多動力同埋support化學友,otherwise,化學友過去一個月一定過得好苦:
2007年5月13日 星期日
2007化學奧林匹克
今年嘅主題係chemistry in commercial products。決賽Present嘅六組同學都搵咗d唔同嘅commercial products去分析同研究: 螢光棒、染髮劑、洗衣液、解酒藥、螺旋藻片同埋驅鳥劑。
講真,六組同學嘅表現幾出色,見到佢哋都花咗唔少心機去準備,係一場高質素嘅比賽。化學友唔想o向e度慢慢講d關於o個日化學嘅內容,反而化學友想講講o個日嘅冠軍St. Paul’s Convent School。其實o個日未宣報邊個係冠軍,化學友都100%肯定St Paul會得到champion o架啦。佢地o個日嘅表現可以話接近完美,勝出係實至名歸。如果同學仔有參加o個以前d chemistry或者science嘅competitions,都會知道St Paul係常客,而每次佢地都會有好好嘅表現。一次成功仲可以話係luck,但每次都有高水準表現就一定係有佢地成功之處。化學友發覺最明顯St Paul比其他學校好嘅地方係佢地每次比賽都可以做到”以終為始”,而一般學校好多時都做唔到。同學仔可能會問乜o野叫做"以終為始" ? 簡單o黎講就係每決定做一件事時,第一樣o野要做就係要定下明確目標先,然後所有過程都係向住e o個目標出發。用St Paul為例,佢地o個project係compare染髮劑,而佢地做嘅每一個experiment或者investigation都係有明確咁為左compare染髮劑而做,唔會左右搖擺。反觀其他學校或多或少都有d守唔住目標,佢地好多時都係為左做實驗而做實驗,而o個d實驗同佢地嘅target係冇乜關係,當d judge問佢地為乜做e o個實驗,同常佢地都唔係好知點解。就係因為有e o個分別,好多時St Paul參加competition都會欏到好成績。老土d咁講,每個人成功都一定有佢嘅優勝地方,絕非僥幸o架。化學友希望同學仔以後參加任可science competition都一定要記住”以終為始”,咁樣你地得到好成績嘅機會就大好多o架啦。(P.S. 同學仔唔好以為化學友o向到同St. Paul賣告白,化學友同St. Paul一d關係都冇o架,只不過化學友真係幾欣賞佢地嘅表現,覺得同學仔參加competition時可向佢地借鏡咋。)
有興趣睇吓Chemistry Olympiad嘅同學仔,可以去e個website睇睇丫:
http://www.hkasme.org 搵 ”HKChO-12th”
2007年5月12日 星期六
曼聯----2007 champion
Yeah! 曼聯必勝!!!!!!
回應
Pinky:
你好,化學友。
我想請問一下如何才能學好chem,
我是指在CE 摘B OR A。
駛唔駛睇多D課外書架CHEM?
化學友:
pinky,你條問題幾難答呀,因為各施各法,好難一概而論。不過化學友發覺自己大部份欏A嘅學生都有一個共通點-----佢地都做得幾多exercise。因為好多時你讀完一d concept,以為自己好明,但係一做exercise,先知自己唔識用o架。多做exercise真係好有用o架,可以令到你溫習事半功倍。坦白講,化學友dF5學生平均每年大約有30% chem欏A,而化學友覺得佢地唔係個個都好聰明,但係好多都係做得exercise多所以先欏到A。化學友深信"practice makes perfect",Pinky你可以試o下e個方法對你有冇幫助呀。
回應2
Wa:
化學友你好...
i would like to ask u one qu abt the chemical reaction in the lemon cell (silver / copper)
1. will copper be dissolved , ie oxidised?...
2. wt would happen if we use nacl soln instead of lemon...?
thx!!!
化學友:
1. Wa, 因為Cu is more reactive than Ag,所以o向lemon cell裡面Cu會lose electron而形成Cu2+:
Cu(s)--> Cu2+(aq) + 2e-
因此,copper會dissolves而變成Cu2+(aq)。仲有因為Cu嘅oxidation number由0變成+2,所以Cu is oxidized。不過化學友覺得你可能未學乜o野叫oxidation number,暫時黎講你可以當一隻element嘅charge增加時(e.g. Cu --> Cu2+),隻element就oxidized,但係當一隻element嘅charge減少時(e.g. Cu2+--> Cu),隻element就reduced。
2. 當NaCl instaed of lemon時,Cu一樣係會dissolve形式Cu2+,因為無論用乜o黎做electrolyte,都唔會影o向anode放電子嘅現象,Cu點都會變成Cu2+。而H2就會o向Ag到形成(至於explanation,你呀sir/miss遲d會教o架啦)。
回應3
ag925:
化學友你好...
i would like to ask u some qus abt a chemical cell using cu/ag as electrodes ...
1. if we use lemon juice as electrolyte ... will copper be oxidized ..?...wt would happen if we used nacl soln as electrolyte...?
化學友:
哈哈.....ag925 你條問題同上面嘅Wa一模一樣o架。你地係唔係同一個人o黎o架?定係你地讀同一間學校??? ag925,如果你唔明o個answer再問化學友丫。
2007年5月7日 星期一
回應
Schoolbag:
有條題我想問xIO3- + yI- + 6H+ ----->_I2 + _H2O
ANSWER:X=1 Y=5
我想知Y可吳可以系3?點解?
化學友:
schoolbag,化學友唸你當Y=3時,以為條equation係IO3- + 3I- + 6H+ --> 2I2 + 3H2O。
其實你睇漏左d o野啦,euqation除左elements要balance, charge都要balance。如果Y = 3,條equation d charge左右唔balance,左面係-2而右面係0,所以唔得呀。明白嗎?
回應2
小白:
多謝你既提醒,我還是複習一下數學吧。因為之前一直都是溫化學物理(其他碰到沒碰)。多謝你肯熱心解答,謝謝。因為我一直都猶疑如果中六是選物理還是選化學好= =
其實中六的化學真的能夠解釋到我們不明白的嗎?即是有些問題老師總是說中六才會教,我怕到時又變成中七才會教,中七問時便是大學才會學。從小學到中學一直都是這樣,有極多問題都是不斷推遲說遲些教,不斷累加心中的疑問,真是非常辛苦。我都沒再對了,因為我本來化學成績就不好,只是臨考前溫了清了一點concept而已。所以能夠合格已經心足了。
化學友:
小白,化學友好明白你嘅疑問(講真,化學友讀F4時都有你嘅唸法),e d o野非三言兩語講得完。咁啦,你會考後有時間化學友再同你慢慢discuss o下啦。
回應3
kino_watashi:
1. 謝謝你的答覆雖然不是很明白你說甚麼但是我在yahoo!知識就找到了關於C-C這樣的一個回覆:True.formation of various (organic) compound is of due to the effective sharing of electron giving a stable covalent bond.(for your reference)the uniqueness of formation long carbon compound is called catenation.this is due to the effective sp3-sp3 sigma overlap of hybrids in C-C and C-H bond, i.e. relative high bond strength and is quite inert to ordinary reaction. therefore, nearly infinitely long or even stable hydrocarbons with cyclic carbon skeleton is formed. chain hydrocarbon is generalized by a formula CnH2n+2still, unsaturated carbon chain can be formed since neighboouring sp2 carbons contributes to effective p-p pi overlap making its formation gives extra stability.compared with silicon(also in group IV), covalent radius of Si-Si is larger. electrostatic attraction between nucleus and bonding electrons are less stronger than that in C-C bond. this is verified by the fact that in Si-Si, sigma overlap is less effective after hybridization. Si can only undergoes catenation up to 5 members
2.我想問下chem mc 42(3) 的amino acid formation 是不是out of syllabus 了?????
如果我chem 85%~90%對, mc 有45/50, 能不能有A?????
3. 我想問下, mc 47是C 而不是D 呢????因為我覺得only one variable 而其他都要the same, 所以我選了D.請指出我concept 上的錯誤, 謝謝~~
化學友:
1. kino_watashi,化學友覺得你chem嘅根底都好好。睇左你forward yahoo!知識段字,其實同化學友嘅explanation同一原理,不過因為e d係AL concept,所以未必睇得明。anyway o個ans係B o架啦。
2. 講真,kino_watashi e條有少少out c喇,因為我地CE多數學嘅monomer係有兩個一樣嘅functional groups,但其實兩個functional groups可以唔同,而都可以做到condensation polymer。e 條其實同CE 2005 條關於PLA 嘅long question一樣,都係有兩個唔同嘅functional groups。
如果你真係得到e o個分就一定A啦,恭喜你呀。
3. mc 47其實好難o架,會考後化學友先答你丫。 add oil la
回應4
Tom:
HIHI~我第一次來的。我對近日教的chem有一些不明,希望你們幫幫手。在simple Cu-Ag cell中,為什麼不可以用CuSO4做electrolyte而要用AgSO4??
化學友:
Tom,CuSO4都可以做electrolyte o架,唔一定要AgSO4。
o向chemical cell裡面electrolyte係幫助conduct electricity,basically任何electroyte(e.g. NaCl)都可以用o向chemical cell裡面o架。只不過唔同electroyte會o向通電後係Ag electrode出唔同嘅products。
例如,
用CuSO4做electrolyte,Cu就會deposite o向Ag electrode
用AgSO4做electrolyte,Ag就會deposite o向Ag electrode
用NaCl做electrolyte,hydrogen gas就會o向Ag electrode evolve
至於點解有唔同 嘅products出,就要用electrochemical series先可以解到。化學友唸你阿sir/miss遲d會教o架啦。
Tom,如果仲有唔明,就留言比化學友啦。
回應5
Eymon:
Dear Sir, one of my classmates said that in one year, only 92% or above correct can get an A in Chem, is it true? In fact, if I get 86% correct in 07 CE, to the best of your experience, do you think that I can get an A or not? Please tell me as soon as possible, since I am very worried and nervous.
化學友:
eymon, sorry呀,咁遲先答你。每一年CE chem mc大約45 out of 50就A,當然有時有例外啦,例如前兩年要o岩成47條先A。但係簡單o黎講每一年on average你mc岩45條long question有73-4分,overall就一定有A。如果你今年mc+long question平均有86%,咁就肯定有A o架啦(e排化學友聽到有d考生話今年分卷容易,要o岩好多先A。化學友肯定咁講一定唔係lor,其實好多考生都唔知自己錯左,以為白己o岩)。唔使worried呀eymon,比心機prepare好d a. maths呀,加油!!!
2007年5月4日 星期五
點點回應
kino watashi:
Thanks~~
1. 我想問下, 為甚麼PVC 可以store concentrated sulphuric acid??? 聽說他們是有reaction 的.假設你mc 10 的答案對了, 為甚麼ethyl ethanoate 不可以store 在 expanded polystyrene container 呢?
2. mc 30 為甚麼第二條statement 不是第一條的正確解釋呢????
請賜教!!~~
化學友:
1. kino watashi,ethyl ethanoate係organic solvent,expanded polystyrene係會dissolve o向ethyl ethanoate。如果有機會,你可以試試將ethyl ethanoate加入expanded polystyrene cup,幾minutes後ethyl ethanoate變成白色,因為polystyrene dissolve左入去ethyl ethanoate到。
至於PVC係inert towards many chemicals例如conc. H2SO4。你可以睇睇2000 Paper II 38就明,PVC唔會同conc. H2SO4 react,佢只會soften at high temp。
2. Carbon can form a large number of compounds with long carbon chains嘅原因係因為C-C bond strong而唔係因為carbon atoms can share elctrons with one another。好多elements(例如Si)都可以share electrons with one another,但係就唔會form compounds with long Si chains因為Si-Si bond好弱。OK ma kino watashi?
回應2
Simon:
可唔可以解釋一下 07 chem mc 既第 5,26,27,39,42同第47題 ? 同埋 .. 我想問 .. mc 幾多分先肯定有C?萬分感激!!
化學友:
Simon,sorry ar,e排都係好忙,d mc遲一排再答你丫。
其實,如果有35條correct就一定有C o架啦,唔使太擔心。
回應3
wk:
你可否評論一下今年Alevel Chem 係深定淺?通常ALEVEL Chem 合格係幾多分?
化學友:
化學友覺得Paper I幾深呀,要考生對chem嘅concept好clear先答得到。Paper II就OK la,都應該可以得到一定分數。
wk,通常ALEVEL Chem有80分(out of 200分)就一定合格o架啦。
回應4
小白:
你好,我之前有問過你問題的,不知道你記不記得。之前上網對了是錯8題,對了你那個是錯了10題。可以解釋下那些問題的答案嗎?4,10*,21,30,31,33,36,38,41,51= ="另外,如果MC錯10題,長題目錯唔超過20分,有冇c = =?我連合格都唔肯定..錯好多....愈對先知愈多錯,一開始睇仲以為岩。
化學友:
化學友更係記得你啦。唔好意思呀,同Simon一樣化學友遲d再答你d mc丫。
小白,其實如果你真係得到e o個分就100%有C或以上,唔使驚呀。仲有呀小白,唔好再對啦,越對得多就會越發覺多錯o架啦,會影o向心情同埋跟住嘅subject嘅performance。相信化學友啦,你chem ok o架啦。
2007年5月2日 星期三
2007 CE mc
1-10:
BDDBDDABDD
11-20:
ADACCBCBAA
21-30:
ACCBDBDCCB
31-40:
AABCDDADAA
41-50:
BDACDBCCBC
anyway,同學仔今日嘅chem已經過去啦,考得好唔好都要比心機prepare Eng同埋Mathso架啦,you should concentrate on your coming exam。(祝聽日要考econ嘅同學有好成績啦)
回應
chemson:
化學友覺得今年LQ,MC幾分先A到?難度怎樣?
化學友:
chemson,今年份卷都幾難。因為要拉curve,所以要改完先知幾多分有A。以住就大約mc 45條(out of 50條) long question 73-4(out of 90分)就A。不過,化學友覺得今年會低d o架啦。
2007年5月1日 星期二
回應(會考化學)
maryuen:
我想知道tin要比copper reactive ma?
化學友:
係呀Maryuen,tin係比copper reactive。
Reactivity series: Fe>Sn>Pb>Cu
回應2
ii:
請問chem ge past paper,98-04年ge mc 同paper1 有邊幾題係out c?thank you
化學友:
98 Paper I Q.3(a), 4, 6(a)(ii)(2)
99 Paper I Q. 7(b)(iv), Q8(b)(i),(iii)
2000 PaperI Q.5, Q.9(c)(ii),(d)
2001 Paper I Q.3(b), Q.8(b)
2002 Paper I Q.1(a), Q.3(a)
2003 Paper I Q.6(b)
2004 Paper I Q.9(c)(i)
98 Paper II 8, 35, 37, 50
99 Paper II 14, 23, 47, 49, 50
2000 Paper II 10, 26
2001 Paper II 13, 39, 48
2002 Paper II 4, 15, 25, 42, 50
2003 Paper II 4, 34, 47
2004Paper II 5, 19, 35, 50
回應3
Chemson:
1. 不好意思,還有一條問題06 LQ 最後果題,determine molar volume marking scheme話用excess HCl我答左excess Mg ribbon有冇分?
2. 我在某本ex.見到條問題,叫我寫equation+observation,chlroine water+iodine solution(i.e.iodine dissolve in potassium iodine)iodine dissolve in potassium iodine即係點?有I2(aq)定I-(aq)?還是兩個都有呢?
化學友:
1. 因為如果用咗excess Mg ribbon,係難d睇得出HCl係唔係完全用晒,因為任何時間都見到有Mg剩,所以呢個寫法唔係幾好,不過都有分。
2. chemson記住I2 dissolved in KI = I2(aq)。因為I2唔係好溶於水,要用potassium iodine增加佢嘅solubility(至於explanation係out c)。
回應4
虫:
除左CaCO3外燒CO3 2- compound既野會唔會出 CO2??
化學友:
會呀,所有metal carbonate都會 give CO2 under heating除左K2CO3同埋Na2CO3。
回應5
Kit:
你好: 我想請問 thermal decomposition的問題,有什麼carbonate salt,nitrate salt,sulphate salt will decompose on heating?Thank you very much!
化學友:
化學友已經係2007年4月12日 星期四嘅回應整咗個表俾同學參考喇,你去see see la。
回應6
Kwok:
1. is heat is needed for the production of NH3 from NH4+ + OH-?
2. 1991年mc第36題why substitution is considered as violent?
化學友:
1. 要呀,會考一定要heat或者warm。
2. 因為呢個reaction係有光情況下都幾勁,好快完成,所以都算violent。
會考化學(14)

2. 各種treat plastic wastes(處理塑膠棄物) method (incineration焚化、landfill堆 田同埋pyrolysis熱解)嘅優、缺點 。可以參考以下嘅pastpaper:
2003 Paper I 5
會考化學(13)
1. 首先要記住e課提及過嘅唔同種類嘅organic compounds(有機化合物):
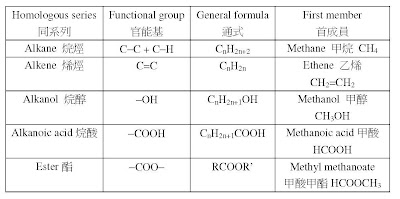
2. 要溫熟cracking process(裂解過程)、reaction condition(反應條件)、equation(方 程式)同埋e個reaction advantages(反應嘅優點) 。睇睇下面嘅pastpaper:
2003 Paper I 7b
3. 記住各種pollutant(污染物)嘅cause(成因)、reducing method(減少方法)同埋 effect on human and environment(對人同環境影響) (注意呀increasing height of chimney (增加煙囪高度)係唔可以減少空氣污染)。 睇睇下面嘅pastpaper:
1999 Paper II 30
4. 注意alkanoic acid(烷酸)同埋ester(酯)嘅關係,它們只係有相同嘅molecular formula(分子式),佢哋係唔同類型嘅compound,有唔同嘅reaction同埋 physical properties(物理性質),例如 b.p.

2007年4月30日 星期一
回應(會考化學)
pele159:
1. 請問 HCl(aq) & HCl(g)係咪compound ?
2. 地球最多的 element 係咪 oxygen ??地球最多的 metal咪 Iron ??
3. conc.H2SO4 + heat 係咪react cu ?Iron heat conc.H2SO4 係會出咩呀 ??
4. 係咪只有HNO3 stored in brown bottles ?
5. 其實而家應該做出面練習的mock 定係 redo 錯嘅野,溫下書好呀 ??
化學友:
1. HCl係covalent compound, (aq)同埋(g)只係代表唔同嘅states。
2. 地球最多嘅 element 係oxygen(之前寫錯,多謝網友指正)。地球最多嘅 metal係 aluminium。
3. Cu + 2H2SO4 --> CuSO4 + SO2 + 2H2O
Iron heat conc.H2SO4:
Fe + 2H2SO4 --> FeSO4 + SO2 + 2H2O
4. 除咗HNO3, 仲有AgNO3同埋KMnO4。CE chem大約知咁多就得o架喇
5. 化學友覺得你應該redo錯嘅野喇,同埋溫番dnote,唔好剩係做題目,會更有效。
回應2
pele159:
1. 86 Q.2The melting points of diamond, sodium chloride and potassium increase in the order:
A. diamond, sodium chloride, potassium
B. potassium, sodium chloride, diamond
C. sodium chloride, diamond, potassium
D. sodium chloride, potassium, diamond
請問 Metallic bond 和 ionic bond , 邊果melting points 大 d 呀 ?
2. 86 Q.38Bromine exists in two isotopic forms, 3579Br and 3581Br . The relative atomic mass of bromine is79.9. Which of the following statements is/are correct?
(1) The relative abundance of each isotopic form is about the same.
(2) The two isotopes have different numbers of protons.
(3) The two isotopes have different numbers of neutrons.
A. (2) only
B. (3) only
C. (1) and (2) only
D. (1) and (3) only
請問(1)點解arm 呀 ?
3. 87 Q.32(1) Nitrogen does not react readily with other elements or compounds.
(2) The outermost electron shell of the nitrogen atom is completely filled.
點解(1) 係arm 呀 ?? 乜Nitrogen 唔係covalent bond ?? 如果要does not react readily with other , 乜唔係 Noble gas 果d咩 ??
化學友:
1. 一般o黎講ionic bond強過metallic bond。所以ionic compound 嘅melting point 係高過metal。
2. 其實當年marking係錯o架。因為79Br有55%而81Br有45%,所以relative abundance is not about the same。
3. nitrogen(N2)內有nitrogen-nitrogen triple bond。化學反應時,需要好高energy才可以break nitrogen-nitrogen triple bond,所以佢係好少reaction,好unreactive。但係佢都有reaction嘅,例如: 3Mg + N2 à Mg3N2。
當然noble gases比N2 unreactive得多啦。
回應3
carmenlee:
你好,我有個chem既問題想問下OCl-會turn red litmus paper blue then white啦;咁H2SO3 turn完blue litmus paper red之後會唔會turn white?p.s.我見d solution都冇寫佢會turn white,但係SO3 2-唔係都bleach到野架咩?
化學友:
carmenlee,你係correct o架。但係SO32-嘅bleaching power比較mild,要long time先可以turn litmus paper white,所以ans只係寫即時observation。
回應4
sinying:
我想問下呢:alkonal加Na,CH3COOH加Na係唔係會都出H2??我睇書話佢地都係會出OH- ion,所以有H2放但咩唔係只係H+ion加Na先會出H2咩?OH-加Na為何出H2,而且CH3COOH唔係ACID來的嗎?咩唔係會出H+...........THX
化學友:
係呀,佢地會出H2 o架:
2CH3CH2OH + 2Na --> 2CH3CH2ONa + H2
2CH3COOH + 2Na --> 2CH3COONa + H2
sinying,化學友唸你睇錯o野喇。凡親一隻compound有H+或者hydroxyl group(-OH group 唔係OH- ion)就可以同Na react to give H2 o架啦。CH3CH2OH有 -OH group而CH3COOH有H+,所以有reaction。
2007年4月29日 星期日
會考化學(12)
1. 連接兩個half-cells(半電池)之間所用嘅salt bridge(鹽橋),目的嘅係容許half-cells內嘅ions(離子)互相通過。 睇睇下面嘅pastpaer:
1995 Paper I 9b
2004 Paper II 40
2006 Paper II 40
2. 好多時卷二都有條計氧化數同埋判斷邊條係氧化還原反應嘅mc,e兩年好多考生都錯,所以要小心。睇睇下面嘅pastpaer:
2005 Paper II 8
2006 Paper II 3
2006 Paper II 27
3. 如果我地想acidify potassium permanganate(酸化高錳酸鉀)或者係sodium dichromate(重鉻酸鈉) 所用嘅acid應該係dil. sulphuric acid (硫酸)。唔用HCl(aq)(氫氯酸) 嘅原因係MnO4-會oxidize(氧化)Cl-(氯離子)。 唔用HNO3(aq)(硝酸)嘅原因係HNO3自已都係1隻oxidizing agent(氧化劑),佢嘅存在會同MnO4-或Cr2O72-爭住oxidize另一隻reducing agent,出現unexpected result。睇睇下面嘅pastpaer:
1999 Paper II 15
4. 比較Fe2+同埋Fe3+嘅difference:
Fe2+
colour: pale green(淺綠色)
+ NaOH: dirty green precipitate
+acidified KMnO4: pale green --> yellow
Fe3+
colour: yellow(黃色)
+NaOH: brown precipitate
+acidified KMnO4: no observable change
5. 解釋reaction係唔係redox, 只需寫出reducing agent(還原劑) 或者係oxidizing agent (氧化劑) 嘅 change of oxidation number (氧化數轉變)。例如,2Na + Cl2 --> 2NaCl係redox,因為嘅O.N. of Na increases from 0 to +1 / O.N. of Cl decreases from 0 to -1。
6. 注意在electroplating(電鍍)裡,anode(陽極)、cathode(陰極)和electrolyte(電解質)嘅配搭。睇睇下面嘅pastpaer:
1993 Paper I 2a
1995 Paper II 11
1996 Paper I 9b
係第六章重要嘅工業產品裡面,同學仔要留意以下幾點:
1. Chlorine(氯氣)溶係NaOH係一個redox reaction(氧化還原反應),而最重要係Cl同一時間 oxidized同埋reduced。
Cl2 + 2NaOH --> NaCl + NaOCl + H2O
Cl嘅O.N.changes from 0 to -1(Cl2--> NaCl)
at the same time Cl嘅O.N. changes from 0 to +1(Cl2 --> NaOCl)
睇睇下面嘅pastpaer:
1997 Paper II 21
1999 年 Paper II 36
2. compare chlorite ion(次氯酸根離子)同埋sulphite ion(亞硫酸根離子)作為bleachingagent(漂白劑)嘅分別:

睇睇下面嘅pastpaer:
1998 Paper II 32
2001 Paper I 9d
2005 Paper II 17
3. Conc. sulphuric acid(濃硫酸)同dilute sulphuric acid(稀硫酸)不同嘅特性。只有conc. sulphuric acid有 oxidzing property同埋dehydrating property,而di. sulphuric acid就冇。
Oxidizing(氧化性) of conc. sulphuric acid:
Cu + 2H2SO4 --> CuSO4 + SO2 + H2O
C + 2H2SO4 --> CO2 + SO2 + 2H2O
S + 2H2SO4 --> 3SO2 + 2H2O
Dehydrating(脫水性) of conc. sulphuric acid:
CuSO4.5H2O --> CuSO4 + 5H2O
C12H22O11 --> 12C + 11H2O
(C6H10O5)n --> 6nC + 5nH2O
4. conc. sulphuric acid同埋dil. sulphuric acid都有嘅相同特性。
同alkali solution(鹼溶液) react:
H2SO4 + 2NaOH -->Na2SO4 + 2H2O
同carbonate salt(碳酸鹽)嘅反應:
H2SO4 + Na2CO3 --> Na2SO4 + CO2 + H2O
睇睇下面嘅pastpaer:
1998 Paper I 8a1999 Paper II 7
2001 Paper I 9c
5. o向計算molar volume(摩爾體積)時,要留意以下兩點
i. 在same no. of moles(相同的摩爾)下,gas嘅體積比liquid同埋solid大好多,所以記住一mole N2同一moleNaCl或者一moleH2O嘅volume係唔同。睇睇下面嘅pastpaer:
1998 Paper II 46
2000 Paper II 48
ii. 留意計diatomic gas molecule(雙原子氣體分子)時,係問atom(原子) 定係問molecule(分子),d考生成日都wrong o架。睇睇下面嘅pastpaer:
2001 Paper II 27
回應(會考化學)
Sinying:
1. 我想問下呢~detergent 的hydrolysis.係唔係有得用H2SO4用NaOH架??兩種有D咩分別??
唔該哂
2.係sacrificial potection 裏,如Zn用來protect Fe,我想問Zn會唔會oxidize 同oxygen 會唔會form around Zn??
3.ZnO + hydroxide ion係會出d咩? ZnO又唔會溶水,有冇REACTION,仲會唔會redissolve架??
超級thanks
化學友:
1. 唔係o架。只有NaOH + fat/oil先會做成soap,H2SO4係唔得o架。你可以睇o下下面化學友回應pele159嘅回應丫。化學友比多兩條equations你睇,可能會更易明:
2. Sacrificial protection 係用Zn 代替 Fe loss electrons變成Zn2+,而oxygen and water react形成OH-,Zn2+會同OH-react生成zinc hydroxide around zinc。
3. e條有少少out c o架。ZnO同Zn(OH)2一樣會redissolve in excess OH-生成Zn(OH)42-
Maryuen:
我想知道關於alcohol的什麼是thinning of blood?
它跟being less rpone to heart attack有什麼關係??
正式嘅解釋化學友都唔敢肯定,大致係指alcohol可以溶解blood裡頭嘅fat,令到blood嘅viscosity降低(即係令d blood冇咁黏)。如果dblood黏,可能會引至血管閉塞,咁就會有heart attack。
回應(會考化學)
pele159:
1. 請問寫essay 洗唔洗寫 equation ??
2. Preparation of sulphuric acid係咪 Contact process ??
3. 如果想整 Conc. Nitric acid 可以點整呀 ?
4. 請問係咪有個新方法整 soap ( acid hydrolysis of fat or oil ) ?
Fat/oil + water <=> fatty acids + glycerol
5. 整soapless detergent 係咪要 conc.H2SO4 & NaOH 呀 ??
6. 你上次答NaOH同hydrolysis裡面形成嘅carboxylic acid neutralize生成salt of carboxylic acid(即係soap) 咁我英文可以點答呀 ??
7. 請問點解 soap 係 alkaline ( 佢又冇OH-ion ) ?
化學友:
1. 寫essay 係唔洗寫 equation,因為essay係考communication skill (傳意技能),以文字表達chemical knowledge。除非係題目要求,平日係唔使寫equation嘅,仲要記住唔好以point form寫出,真係會扣分o架。
2. 係呀,整sulphuric acid嘅industrial process係指Contact process(接觸法)。
3. 整conc. nitric acid嘅industrial process已經out c喇,不過都俾你參考吓啦!
首先係catalytic oxidation of ammonia (氨嘅催化氧化),用Cu or Pt做catalyst。
4NH3(g) + 5O2(g) -->4NO(g) + 6H2O(g)
跟住NO再同空氣中嘅O2react生成NO2
2NO(g) + O2(g) --> 2NO2(g)
最後dissolve NO2落水就製成HNO3
4NO2(g) + O2(g) + 2H2O(l) --> 4HNO3(aq)
4. 唔係呀pele159,fatty acid係唔同soap,fatty acid係 carboxylic acid,佢唔溶於水。而soap係ionic compound,佢溶於水先至有清潔效能。化學友估你可能同ester 嘅acidic or alkaline hydrolysis撈亂咗,無錯fat/oil係ester,佢哋係可以carry out acidic or alkaline hydrolysis。但係當fat/oil carry out acidic hydrolysis,就只會出咗隻唔溶於水嘅fatty acid,呢隻compound係唔會有cleaning power。如果fat/oil carry out alkaline hydrolysis,就會出soap。
5.係呀,petroleum fraction + conc. H2SO4,然後加入NaOH,就會做成soapless detergent。6. pele159,睇吓咁樣寫O唔OK: NaOH neutralize the carboxylic acid formed to give sodium salt of carboxylic acid which is the soap.
7. 因為當soap溶咗落水,佢會同水有化學化應生忒OH-。雖然條equation out c,但係都寫比你睇下啦: RCOO- (soap) + H2O <=> RCOOH + OH-
回應2
Maryuen:
1. 化學友,我想知道怎麼看CH3CO2H->CH3CH2OH的oxidation no.?? oxidation no.是什麼??
2. 1998 5: how to use the test tube given? wt is the answer in the marking scheme?
3. 7aiii3: why ethanol can dissolve unpolymerized monomer (vinyl chloride)?(joint us answer)
4. 7bii3: PVC is not suitable for making electric socket since the current pass through it will produce heat and melt it??
5. 9aiii: how to write the equation with [O] for the reaction between propan-1-ol and acidified potassium dichromate solution?
6. 9avi1: State TWO observable changes when the contents of the test tube (ester) were added to the sodium carbonate solution.
can i write a thin colourless layer form on top of the sodium carbonate solution??
化學友:
1. 要計到e d oxidation no. 需要d F.6 嘅concept。 化學友記得e條係pastpaper mc,其實你可以simple d 咁睇: CH3CH2OH --> CH3COOH (ethanol --> ethanoic acid)係oxidation,所以CH3COOH --> CH3CH2OH就係相反嘅reduction。
2. d test tubes 係用o黎裝d chemicals嘅
3. 因為vinyl chloride同埋ethanol都係organic compounds,化學友唸你老師應該教過'like dissolves like'嘅concept,所以ethanol可以dissolve vinyl chloride。
4. 正確d o黎講係防止火警時高溫會 melt 咗d PVC,咁copper wire外露會非常dangerous,引至explosion或漏電。
5. maryeun,你係唔係想問e絛equation: CH3CH2CH2OH + [O] à CH3CH2COOH ?
6. 係可以o架,因為ester係insoluble in water,而且浮o向水面。
2007年4月28日 星期六
回應(會考化學)
Yin ni:
我mc卡住係30-35 ge range
係呢3日有咩方法可以提升呢?
做左97-05pp 了
做多次有效嗎?
化學友:
yin ni,30 - 35條mc大約得到C-D grade。化學友suggest你將所有錯嘅mc再做多幾次(唔使全部再做,冇咁多時間o架啦),你應該會發覺成日都係錯類似嘅o野,o個d就係你嘅弱項,會令你mc一路冇乜進步。主攻改善弱項應該會令你有明顯嘅improvement。跟住去明報o個website download幾份mock做practice丫:
http://life.mingpao.com/htm/hkcee2007/cfm/main1.cfm
化學友覺得咁樣會最effective。盡力啦yin ni,化學友以前有d學生mc都係卡住o向30-35,但係佢地跟住化學友講嘅咁做,最後都得到B。
回應2
Cathy:
我想問有關1998年既paper1既6ai/2
唔係standard solution放係burette到咩???我想知邊樣放係burette同conical flask呢???
化學友:
Cathy,standard solution一定要放o向burette到係好多年前嘅做法,e家standard solution可以放o向burette或者conical flask都得o架。
回應3
小白:
您好,有些問題想問您:
Q1在會考中,泡騰現象是否專來形容生成二氧化碳氣體。
Q2Ca(OH)2,CaCO3,它們是微溶於水,但寫化學式時卻有時是(s),有時是(aq)....怎麼區分。還有...是不是只有Mg跟Ca會微溶於水??
Q3:除了氮外還有甚麼氣體不溶於水,所有貴氣體?(會考程度)
Q4:溴與碘溶液作電解時,只有極稀濃度時才不會優先放電嗎?還是說稀時也不能= ="?
Q5:鍚還是鐵活潑?錫與鋅還有甚麼分別(除了鋅有毒),因為做了一題MC說鍍錫不宜作防止鐵船生銹,但鋅卻可以。
Q6:為甚麼肥皂容易被生物降解,不是說只有沒有支鏈才容易被生物降解嗎?但肥皂必定沒有支鏈的嗎?
Q7:金屬氧化物加熱能夠生生成氧氣的就只有汞(II)嗎?銅的煅燒跟直接加熱有甚麼不同嗎?
化學友:
1. 唔係呀。凡親一個reaction出好多gas bubbles就叫做泡騰(effervescence)。所以Ca+ 2H2O ---> Ca(OH)2 + H2 都可以叫泡騰。
2. 小白,其實(aq)同埋(s)都correct。如果你e家覺得唔知幾時寫(aq)或者(s),化學友suggest你CE chem時唔好寫state symbol,因為唔會扣分o架。
Metal當中只有K, Na同埋Ca會溶水(佢地同水react產生metal hydroxide,而metal hydroxide溶o向水到),其他metal唔會溶水。
3. H2, N2, CO同埋所有noble gases(貴氣體):唔溶水
O2, CO2, Cl2: 少少溶水
4. 其實你做街外買嘅exercise時,有時唔知幾時會出I2幾時會出O2。但CE chem會好明顯: conc. NaI就出I2,至於very dil. NaI就出O2。dil. NaI就有時出I2,有時出O2,但條question會明示想你寫邊o個答案。
5. Zn(鋅) is more reactive than Fe而Fe is more reactive than Sn(錫) (活潑性:Zn>Fe>Sn)。
因為防止鐵船生銹係用sacrificial protection(犧牲性保護),所以一定要用一隻活潑過Fe嘅metal例如Zn做protection。
6.小白你完全correct呀。只有冇支鏈嘅先容易被生物降解,而所有肥皂一定冇支鏈o架。
7. 仲有Ag2O遇埶會生成氧氣呀(2Ag2O --> 4Ag + O2)。Cu直接加埶生成CuO (2Cu + O2 --> 2CuO)。而Cu metal冇煅燒o架,煅燒(roasting)係形容化合物o向空氣度加熱,Cu metal係elemnt,所以冇煅燒。
明白嗎小白?
會考化學(11)
1. Ca放在進水裡嘅observation: Ca沉於水及有colourless gas釋出,記住絕無brick-red flame(磚紅色火焰),只有Na同埋K遇水先會有yellow同埋lilac flame。留意下面嘅pastpaper:
200o paper I 9a
2003 paper I 2
2004 Paper I 1
2000 Paper II 33
2. 會考寫equation唔使寫state symbol,唔會扣分。(除非條題寫明要寫state symbol)
3. Anodized aluminium(陽極氧化鋁)只會加強corrosion resistant (抗腐蝕 性),並唔會加強佢嘅strength。同埋鋁嘅usage係同佢係最高蘊藏量無關。 留意下面嘅pastpaper:
1993 Paper I 1a
2001 Paper I 5
2005 年 Paper II 48
係第四章Acid and base(酸和鹼)裡面,同學留意以下幾點:
1. 測試ammonia(氨)的方法:NH3能將moist red litmus paper(濕潤的紅色石蕊 試紙)轉為blue(藍色)。留意下面嘅pastpaper:
2005 年 Paper I 12
2006 年 Paper I 4
2. Metal ions+ hydroxide ion (from NaOH或者NH3) --> precipitation 。e種reaction叫做precipitation reaction(沉澱反應)
Equation Colour of precipitate
Zn2+(aq) + 2OH-(aq) --> Zn(OH)2(s) white
Pb2+(aq) + 2OH-(aq) --> Pb(OH)2(s) white
Al3+(aq) + 3OH-(aq) --> Al(OH)3(s) white
Cu2+(aq) + 2OH-(aq) --> Cu(OH)2(s) blue
Fe2+(aq) + 2OH-(aq) --> Fe(OH)2(s) dirty-green
Ni2+(aq) + 2OH-(aq) --> Ni(OH)2(s) bluish-green
Fe3+(aq) + 3OH-(aq) --> Fe(OH)3(s) brown
Cr3+(aq) + 3OH-(aq) --> Cr(OH)3(s) green
仲有Al(OH)3, Zn(OH)2 and Pb(OH)2 dissolve in excess NaOH,而Zn(OH)2同埋Cu(OH)3 dissolve in excess NH3。留意下面嘅pastpaper:
2001 年 Paper I 2b
2003 年 Paper I 8b
2005 年 Paper I 4c
3. Cu同alkanoic acid(烷酸)、dilute hydrochloric acid(稀氫氯酸)同埋dilute sulphuric acid (稀硫酸)係冇化反應o架(d考生一考試就成日錯o架啦)。留意下面嘅pastpaper:
2005 年 Paper I 3b
2005 年 Paper II 38
4. Acid-base titration (中和滴定) 嘅mole calculation (摩爾計算題)同埋 experimental procedure(實驗過程)。差唔多年年都考。留意會考化學(7)。
5. 當然仲有rate of reaction啦,今年大機呀。留意會考化學(7)。
會考化學(10)
係第一章大氣(atmosphere)裡面,同學會對熔點、沸點與物質三態嘅關係都會容易混淆,所以係會考成日出o架。
熔點(melting point)係指物質由固態轉成液態嘅溫度(solid --> liquid)。如果果樣物質嘅熔點係高過25°C,佢就係固體o黎。
沸點(boiling point)係指物質由液態轉成氣態嘅溫度(liquid --> gas)。如果果樣物質嘅沸點係低過25°C,佢就係氣體o黎。
如果25°C係位於果樣物質嘅沸點同熔點之間,佢就係液體o黎。
你可能會覺得化學友咁淺嘅o野都講,有冇攪錯呀。但係化學友發覺每次考e樣o野,d同學仔就勁錯wor。想知自已識唔識,就做下面幾條mc丫,其中有d係奸奸地:
1. 2004 PaperII 29
2. 2005 Paper II 3
3. 2006 Paper II 6
另外一樣要留意就係問過裝石灰水Ca(OH)2果樽口果d白色固體係咩黎? 答案就係CaCO3。係由於石灰水同空氣嘅CO2反應而成(Ca(OH)2 + CO2 -->CaCO3 + H2O)。睇吓e條mc 2004 Paper II 11。
至於第二章Microscopic world(微觀世界)裡面,同學仔記住留意下面幾點:
1. Definition of isotopes(同位素定義) - Atoms of same element which have the same number of protons but they have different number of neutrons. (相同元素內的不同原子,它們之間有相同的質子但有不同的中子數目。)
2. 留意1998 Paper II 1同埋2002 Paper II 1,關於electronic arrangement (電子排佈),有d tricky。
4. 留意一d平日o向書本甚少見到嘅化合物嘅電子結構圖畫法。例如:S2Cl2 and H2O2(d考生成日畫錯e兩個圖o架:

2007年4月27日 星期五
會考化學(9)
會考化學(8)
今次化學友想同同學仔講吓paragraph length(段落式) question(一般人叫e d題目做essay),大家一定要對佢嘅出法要有d認識。同學仔做過05同埋06pastpaper都知道卷一有兩條呢d題目,一條出自核心部份而另一條出自延展部份。一般o黎講每條佔9分,6分係內容分而3分係傳意技能(communication)。
通常6分內容分即係要答6個points,記住at least要寫到6個points先好停手。仲有訝chem essay唔使要求你寫引子同埋結尾o架,所以唔好waste time去寫呀‧
至於傳意技能嘅3分係依據以下條件俾分:
1分係俾以paragraph length寫出(即使唔係用point form或者table,而係一段一段咁寫出o黎)
另1分係俾文章嘅前文後理通唔通順(如果用english寫essay,accuracy of using english都計埋o架)
最後1分係俾無化學知識上嘅失誤(例如你寫combustion of fuel in factories會產生CO2, SO2,而CO2 同埋SO2會引至global warming就會冇左e一分,因為SO2係唔會引至global warming)
至於今年化學友嘅熱門心水題包括
核心部份:
(i) Metal extraction (金屬提取);
(ii) Neutralization (中和作用);
(iii) Preparation of chlorine bleach and its reactions (氯漂白水製備及反應);
(iv) Preparation of sulphuric acid and its reactions (硫酸製備及反應);
(v) Distinguish different types of organic compounds (辨別不同類型有機化合物)
延展部份:
(i) Aluminium corrosion and anodization (鋁的腐蝕及陽極氧化);
(ii) Design experiment related to titration (設計與滴定法有關實驗);
(iii) Design experiment on rate of reaction (設計反應速率實驗)
(iv) Compare and contrast of soapy and soapless detergent (比較皂性與非皂性清潔 劑)
最後要remind同學仔:
1. 寫essay要用complete sentence。
2. 盡量唔好寫symbol或者formula,要寫全名,例如唔好寫C同埋NO要寫carbon同埋nitrogen oxide。
3. 一般唔好用多過十分鐘寫一條essay,你會冇時間做其他題目o架。
回應(會考化學)
Maryuen:
1. 我還想知道dilution of acid/alkali是不是都是exothermic?而dissolve NaOH pellets in water呢??can you explain?
2. polyvinyl chloride是可以用來make electrical insulaton for wires,但它不是thermosetting plastic,這樣都可以嗎??
3. wt is topax?(out c?)wt is monosodium glutamate?(out c?)wt is bronze?(out c?)
4. 是不是all non-metal produce acidic oxide?
化學友:
1. Yes ah。diliuting conc. acid、conc. alkali或者NaOH pellets都係exothermic (一般會考唔會問diluting dil. acid或dil. alkali,因為可能冇熱放出。所以考試一定係diluting conc. acid and conc. alkali)。
至於explanation呢就真係out c,要o向AL chem先會學。CE 程度好難解釋,而且唔會考o架。
2.係可以o架。因為thermosetting plastic好hard同埋rigid (因為有cross-link),如果用佢o黎做insulation for wire,咁條電 線咪唔彎曲得同埋轉彎lor。所以insulation layer for wire都係要用thermoplastic o黎做,因為佢冇咁hard同埋rigid。
3. 冇錯呀,全部都out c o架。
4. 大部份non-metal oxides都係acidic,例如,CO2, SO2, SO3, NO2, Cl2O....etc。但係有小部份唔係,例如CO同埋NO就係neutral而唔係acidic。
(P.S. CO, CO, SO2, SO3, NO, NO2, Cl2O e幾隻oxides係CE常見嘅non-metal oxides)
OK ma maryuen?
2007年4月26日 星期四
回應(會考化學)
Maryuen:
(6)2004 past paper 9cii2can i use water?
(7)What is duralumin?
(8) 2003 past paper 3biwhat is formula unit stated in the marking scheme?
(9) HBr and conc. H2SO4是什麼reaction?
(10) conc. H2SO4和sugar的reaction observation要write "the heat produced expand air inside the black carbon and rise it up"嗎??
(11) conc. H2SO4 used in the preparation of HCl and HNO3 acid out c了嗎??
(12) H2SO4 ionizes in water to give H3O+(aq) ions??
(13) H2SO4的half equation到底是什麼??
化學友:
(6) 唔得o架,一定要H+。因為只有H+先會react with RCOO- (RCOO- + H+ --> RCOOH)
(7) duralum係由aluminium做成嘅alloy(合金),佢比aluminium more corrosion resistant同埋stronger。
(8) e o個term可以唔使識o架,其實你唔使學佢咁做,用你平日計mole嘅方法做就得o架喇。
(9) maryuen,你o向邊度見到e o個reaction? 照計out out地syllabus。不過寫比你睇下:
2HBr + H2SO4 --> Br2 + SO2 + 2H2O
e o個係redox reaction(因為Br嘅O.N.由-1 change to 0; 而S嘅O.N.由+6 change to +4)
(10) 一般會考答案唔係咁寫,你只要寫成咁就得喇:
i. sugar chars (sugar turns from white to black)
ii. misty fume(H2O(g) is given out
(11) out左喇
(12) 你可睇o下下面回覆pele159嘅答案,你地問緊同一樣o野呀。
(13) H2SO4 + 2H+ + 2e- --> SO2 + 2H2O 或者 4H+ + SO42- + 2e- --> SO2 + 2H2O
第一條accurate d 但會考兩條都接受。
回應(會考化學)
pele159:
H3O+(aq) ions = H+ ion ?
化學友:
pele159,你係唔係o向2003 Paper I Q4嘅marking見到H3O+ e o個答案呀?其實H3O+係同H+一樣o架。你o向CE考試寫H+就得喇,H3O+ out of syllabus o架。
回應2
maryuen:
你好!化學友~之前,我問過你一些問題~可是你還未回應我~我現在多post一次,麻煩你了~
(1)就是SiO2是一個giant covalent compound嗎??如果是,那為什麼沙都是一顆顆十分細小的?它跟SO2的分別又是什麼??
(2)2004 past paper 2awhy the marking scheme say that table salt gives misty fumes?
(3)2004 past paper 6aivhow to deduce the formula?好像很難的樣子..
(4)2004 past paper 8ciiwhy rinsing the mouth can lower the concentration of ethanol in breath?
(5)how to explain that the metal is malleable in term of its structure
化學友:
maryuen, sor 呀,因為化學友o向學校好多o野做,而又幫緊d F5學生prepare CE chem,所以未有時間答你d問題,e家答住你部份問題先喇。
1. maryuen我地唔會叫SiO2做giant covalent compound,咁寫o向會考有機會扣分o架,我地應該叫佢做compound with giant covalent structure。冇錯,理倫上每粒Si同埋O atom都係用covalent bond連埋,而隻compound嘅size應該冇窮冇盡咁大。但係e d 只係理倫o黎,世界上有好多種原因e.g. weathering會將compound with giant covalent structure變細。
SO2同SiO2唔同,SO2係由molecules組成,molecules之間有van der Waals forces,而S同O之間係covalent bond。所以SO2係compound with simple molecular structure。
2.因為發生左下面o個reaction:
NaCl + H2SO4(conc) --> NaHSO4 + HCl
white fume
e o個reaction有少少out of syllabus,不過都值得知道嘅。
3. Water (H2O) is an oxide of hydrogen. Electrolysis of water in the presence of sulphuric acid gives hydrogen and oxygen in a volume ratio of 2 : 1.
(i) Suggest suitable electrodes to be used in the electrolysis.
(ii) Write the half equation for the reaction at the cathode and that at the anode during the electrolysis.
(iii) What is the function of sulphuric acid in the electrolysis?
(iv) Is it possible to deduce the formula of water from the results of the electrolysis? Explain your answer.
其實由(ii)嘅答案,你可知道how to deduce:
anode: 4OH-(aq) --> O2(g) + 2HO(l) + 4e-
cathode: 2H+(aq) + 2e- -->H2(g)
咁即係話如果有4粒e-經過circuit,就會產生一粒O2同埋兩粒H2(即係H2:O2 = 2:1)。d H2同埋O2都係由水o黎,所以H同埋Oo向水裡面嘅比例都係2:1,而formula就係H2O。
4. 原本口裡面有ethanol,rinse之後將水吐出,口裡面ethanol嘅amount就少左好多。咁樣就冇乜ethanol由口腔去到鼻腔,所以concentration of ethanol in breath decreases。
5. Metals can be considered as making up of positive ions and a ‘sea’ of delocalized electrons. The attraction between themetallic ions and the delocalized electrons holds the particles together (metallic bond). Metallic bond is non-directional. Layers of atoms can easily slide over each another. Hence, metals are malleable.
就算會考問你why metals are ductile,答案一樣。
2007年4月24日 星期二
回應(會考化學)
pele159:
你好 我有少少問題
1. 請問 detergent 有 branch 係咪唔 可以溶在水 ??不過點 g 稱為有 branch ??
2. solid citric acid 點解可以 turn moist blue litmus paper red ??
3. 請問整soap 中的 NaOH/H2SO4 係咪catalyst ??我呀sir 話係,不過我問同學,佢地又say no
4. Refer to 03 第9(a)題如果the inert electrode A is copper plate , the B is zinc rod .Sulphur is ZnSO4, sodium is CuSO4 In what the direction would the sulphate ion pass through the porus pot ? Explain your ans .
你可否比一比你個e-mail 我 ??我有圖但唔識 upload,問唔到 ....
化學友:
pele159,答住你部份題目先啦,因為而家畫唔到圖呀。
2. 因為moist blue litmus paper上面有水(即係moist),部份solid citric acid dissolve o向d水到give out H+ ion,所以moist blue litmus paper turns red。
3.pele159,製造soap係要用NaOH(但係唔使用H2SO4;H2SO4係用嚟做soapless detergent)。NaOH有兩個作用:
i. as a catalyst in the hydrolysis of fat/oil
ii. 同hydrolysis裡面形成嘅carboxylic acid neutralize生成salt of carboxylic acid(即係soap)。
所以你阿sir係啱o架。
4.如果好似你咁講,ZnSO4(aq)就會越嚟越多Zn2+,因為Zn electrode gradually dissolves to give Zn2+(aq),即係e部份嘅 electrolyte越嚟越+ve(因為Zn2+多左,但係SO42-冇多到冇少到)。而CuSO4(aq)就會越嚟越少Cu2+,因為Cu2+(aq) deposites on the Cu electrode,即係e部份嘅 electrolyte越嚟越-ve(因為Cu2+少左,但係SO42-冇多到冇少到)。為左中和d charge,SO42-就會由CuSO4 move to ZnSO4,令到兩面嘅electrolytes唔會太+ve或者-ve。明唔明呀?
pele159,你可以send幅圖去e嗰email丫: friendium@gmail.com
chemistry in action(AL10)
Polymers有d例子係會考讀過,但係更加要留意新加嘅例子,例如,Kevlar。而且同學仔特別要留意effect of structures on different polymers e一部份,要識嘅o野包括high density polyethene同埋 low density polyethene; nylon同埋 Kelvar; vulcanization of rubber ; biodegradable plastics。 精選pastpaper:
1. 1994 PaperII 9b
2. 2004 PaperII 7a
Drugs就主要學aspirin同埋 cis-platin嘅 five stages of drug development。其實e part冇乜精選pastpaper,不過都可以做o下下面兩條:
1. 1998 paperII 6c
2. 2006 Paper II 5b
至於Green chemistry梗係 要識12個green chemistry嘅principles尤其是atom economy係大熱。Specific examples 就有decaffeination using supercritical CO2同埋 use of H2O2 in the presence of manganese based catalyst as bleaching agent。如果仲有時間可以去e個website睇吓一個green chem有關嘅 experiment:
http://www.chem.cuhk.edu.hk/S6_ResourceBK.htm(選第12個實驗 - Green chemistry: an environmentally friendly preparation of 1,6-hexanedioic acid 綠色化學:1,6-己二酸的一種環保製法。有機會考o架。)
考評局出o個份sample paper都有 d同e個topics有關嘅questions:Paper I Q.6(a) - Polymers; Paper II Q.5(c) - Drugs, Paper II Q.7(c) - Green chemistry。如果仲有時間就做下啦。
同學仔呀,e一個月化學友寫嘅嘢,真係希望對你地o向AL有d幫助啦。衷心祝你地成功呀。如果你地覺得化學友o個blog對讀chem嘅弟弟妹妹親戚朋友師妹師弟男朋友仔女朋友仔....etc有用嘅話,叫佢地多d o黎睇下丫,化學友會盡力寫多d好o野o架喇。
最後,祝你地星期四打敗AL Chem e隻怪獸啦:
回應(AL)
hahayi2007:
1. 你之前有題mechanism 有無答案?是不是HONO2 的OH的O打落去H2SO4個H到,甩左水,因為H2SO4係more acidic than HONO2跟住NO2+再比double bond of benzene attack,跟住就照做落去..
2. 我想問下..atom economy如果有隻reactant 係in excess,咁係計formula mass of reactant定係mass of reactant?thx.
化學友:
1. hahayi2007,其實o個mechanism寫到下面咁就滿分o架啦:

2. 任何時間都係用formula mass of reactants o黎計o架。
d block elements(AL9)
同學仔要知道ligands嘅 relative strength:monodentate multidentate ligands (EDTA)。但係其實佢哋都係舊瓶新酒,e d內容係兩代前嘅syllabus都有o架啦。
同學仔,其實d block elements有好多值得做嘅pastpaper,不過化學友都盡力精選左幾條出o黎,希望精益求精啦:
1. 1992 Paper II 4a
2. 1996 Paper II 6b
3. 1998 paperII 8a
4. 1999 paper II 2d
5. 2000 PaperII 2c
6. 2003 PaperII 2b
2007年4月23日 星期一
回應(會考化學)
pele159:
你好 我有少少問題
1. 請問 detergent 有 branch 係咪唔 可以溶在水 ??不過點 g 稱為有 branch ??
2. solid citric acid 點解可以 turn moist blue litmus paper red ??
3. 請問整soap 中的 NaOH/H2SO4 係咪catalyst ??我呀sir 話係,不過我問同學,佢地又say no
4. Refer to 03 第9(a)題如果the inert electrode A is copper plate , the B is zinc rod .Sulphur is ZnSO4, sodium is CuSO4 In what the direction would the sulphate ion pass through the porus pot ? Explain your ans .
你可否比一比你個e-mail 我 ??我有圖但唔識 upload,問唔到 ....
化學友:
pele159,答住你部份題目先啦,因為而家畫唔到圖呀。
2. 因為moist blue litmus paper上面有水(即係moist),部份solid citric acid dissolve o向d水到give out H+ ion,所以moist blue litmus paper turns red。
3.pele159,製造soap係要用NaOH(但係唔使用H2SO4;H2SO4係用嚟做soapless detergent)。NaOH有兩個作用:
i. as a catalyst in the hydrolysis of fat/oil
ii. 同hydrolysis裡面形成嘅carboxylic acid neutralize生成salt of carboxylic acid(即係soap)。
所以你阿sir係啱o架。
4.如果好似你咁講,ZnSO4(aq)就會越嚟越多Zn2+,因為Zn electrode gradually dissolves to give Zn2+(aq),即係e部份嘅 electrolyte越嚟越+ve(因為Zn2+多左,但係SO42-冇多到冇少到)。而CuSO4(aq)就會越嚟越少Cu2+,因為Cu2+(aq) deposites on the Cu electrode,即係e部份嘅 electrolyte越嚟越-ve(因為Cu2+少左,但係SO42-冇多到冇少到)。為左中和d charge,SO42-就會由CuSO4 move to ZnSO4,令到兩面嘅electrolytes唔會太+ve或者-ve。明唔明呀?
pele159,你可以send幅圖去e嗰email丫:friendium@gmail.com
Group IV(AL8)
e個topic有3個重點:
1. Interpretation of variation in melting point and boiling point of the elements in terms of structure and bonding (e個group由carbon係non-metal 到 silicon and germanium 係 semi-metals 到最後tin and lead 係metals)
經典pastpaper: 1993 Paper II 4b
2. decomposition of group IV oxides and chlorides (同學仔要知道group IV嘅compounds有兩個possible 嘅oxidation states +4同 +2;+4 嘅oxidation states stability decrease down the group; 而+2 oxidation state 嘅stability increase down the group; 佢嘅原因可以用inert pair effect 去解釋)
經典pastpaper: 1992 paper II 5b同埋1994 paper II 6
3. silicon and silicates,e part係全新內容,冇pastpaper。但係化學友發現教統局製作咗一份reference material可以幫助同學仔溫習,你地可以去e個網址睇吓:
http://cd1.emb.hkedcity.net/cd/science/chemistry/s67chem/chem.htm (click L&T activities然後o向other learning activities就會搵到Silicates 硅酸鹽嘅notes )
重點係留意silicate嘅不同嘅basic units。
Organic chem嘅mass spect同埋acids & bases(AL7)
2002 Paper II Q.1(b)
如果同學仔平日冇乜做過e d題目,化學友就highly recommend你地去eo個網址睇o下(d questions嘅教統局同中大化學系合作出嘅),非常有用o架:
http://cd1.emb.hkedcity.net/cd/science/chemistry/s67chem/chem.htm (click 'MS'就得o架喇)
Total有七條題目,Q.1-3 同R+有關; Q.4-5同RCO+有關; Q.6-7同C6H5CH2+有關。
另外係課程仲加咗兩個topics,佢哋係 organic acids and organic bases 同埋redox reactions。如果同學仔小心d對吓,其實佢哋係都係將舊syllabus重組過,有好多pastpaper關於e兩個topics。化學友比幾條好有用嘅pastpaper同學仔丫,做熟佢啦:
1. 1999 Paper II 6a
1. 2000 Paper I 6a
2. 2001 Paper II 6b
3. 2003 Paper I 6a
4. 2004 PaperII 6c
最後化學友d學生成日問iodoform test, silver mirror test, diazonium salt 係唔係 out of syllabus,係道順便同大家講埋,無錯佢哋係已經唔洗識o架啦。
加油呀同學仔!!!!
mechanism2(AL6)
其實化學友覺得e個mechanism好有用,佢能夠幫助同學仔明白點樣可以將carboxylic acid 變成佢嘅derivatives。課程指定嘅example係hydrolysis of acyl chloride。其他嘅examples就有 carboxylic acids react with thionyl chloride, alcohols, ammonia and amines同埋 acyl chlorides react with alcohols, ammonia and amines。
以下就係 hydrolysis of ethanoyl chloride 嘅mechanism,大家要記熟呀:

同學仔亦可以睇o下下面嘅simple animation丫,會令你容易d記o架:

2007年4月22日 星期日
回應(會考化學)
maryuen:
1. 我想知道什麼時候有depolymerizaton and 它是怎麼發生的??(有沒有example,會考有沒有機會出??)
2. 另外..2001 past paper 5那個anodization要不要寫eletrode的名?? sacrificial protection要寫example嗎??是不是所有essay都要有引入??
化學友:
1. maryuen,其實depolymerization o向近年嘅CE chem都好少好少出o架,化學友覺得就算出都會好容易嘅。你只要當佢係polymerization嘅相反就得o架o拉。例如:

睇到嗎maryeun o個reaction只係polymerization嘅相反。至於reaction conditions,一般都係high temperature同埋in the absence of air(否則d polymer會burns) 。
2. 2001 Paper I 5 : Explain why anodization, sacrificial protection and tin-plating can protect metals from corrosion. (9 marks)
maryuen,其實寫唔寫都冇問題o架,因為anodization一定係將aluminium做anode,所以唔都ok。2005嘅marking就冇要求你寫example,但係有時你唔知邊一年marking要寫example,所以化學友suggest你o向考試寫埋啦。CE chem嘅essay係唔使寫引子o架,一般9分嘅題目你寫夠6個points(6分)就滿分o架啦(其餘3分就係communication分)。關於essay嘅detail information,遲一、兩日再講比大家知丫。maryuen唔明再問我啦。
回應2
sinying:
1. 唔好意思........我想問ionization 同埋dissolve in water係點分架?HCL 係dissolve water 定ionize???咁NACL呢?
2. 我仲想問呢....Al2O3都係dehydrating agent 來的嗎?仲有d咩compond 係dehydrating agent呀??THX!!
化學友:
1. sinying,化學友唸你對一隻substance dissolves in water嘅方法唔係太了解。其實一隻substance dissolves in water係有唔同嘅途徑,下面係CE chem常見嘅,例如:
i. sugar溶落水。sugar只係simply dissolves in water,佢同水冇化學反應,溶之前佢係exist as sugar molecule,溶之後佢都係exist as sugar molecule。
ii. Na(s)溶落水。Na加落水時,Na同水產生化學反應生成NaOH(s)。而NaOH(s)係溶o向水to give NaOH(aq),所以Na溶水係因為佢同水有化學反應。
iii. NaCl(s)溶落水。NaCl(s)裡面o個d Na+ ion同埋Cl-ion溶之前係 not mobile嘅,溶之後Na+ ion同埋Cl-ion變左mobile,同sugar一樣佢同水冇化學反應。
iv. HCl(g)溶落水。HCl(g)溶之前佢係exist as HCl molecule,溶之後發生ionisation:HCl --> H+ + Cl-,而H+同埋Cl-都係溶落水嘅。
簡單o黎講dissolve in water同ionisation冇乜關係,只不過有時一隻substance溶落水會發生ionisation咋。好似HCl咁,佢溶落水時會發生ionisation。
2. 凡親一隻substance可以將某一compound裡面嘅H同O remove 變成H2O, 佢就可以叫做dehydrating agent。CE chem syllabus裡面當我地剩係知遒conc. H2SO4係dehydrating agent,其他o個d dehydrating agent係唔使記,只要我地o向考試時見到隻substance可以將一隻compound嘅H2O remove,佢就係dehydrating agent o架喇。例如,

根據上面嘅定義,conc.H2SO4, Al2O3同埋P2O5都係dehydrating agent。唔使死記o架。答唔答到你呀sinying?
2007年4月21日 星期六
mechanism(AL5)
1. alkane嘅free radical substitution
2. alkene嘅electrophilic addition
3. haloalkane嘅necleophilic substitution (SN1 and SN2)
4. carbonyl compound嘅nucleophilic addition
新加嘅就有
1. benzene嘅electrophilic substitution
2. carboxylic acid and its derivatives嘅nucleophilic acyl substitution。
其實e兩個mechanisms係前兩代嘅syllabus已經有o架啦。只不過係舊瓶新酒o者。
每一年AL exam都要同學仔寫其中一、兩個mechanisms,當然今年新加o個兩個就最大機會啦。所以化學友想講下e兩個mechanisms。首先,講嘅係benzene嘅electrophilic substitution,課程指定嘅example係mono-halogenation of benzene。 以下就係mono-chlorination of benzene嘅mechanism,同學仔一定一定要記熟呀:

下面係化學友做嘅simple animation,睇o下對你有冇用丫(按下先睇到):

教統局都有做咗幾個mechanisms嘅animations幫同學仔學習,可以去e個網址睇吓,做得幾好o架:
http://cd1.emb.hkedcity.net/cd/science/chemistry/s67chem/reaction_mechanism_animation_e.swf
最後就係俾條題目大家唸吓,考吓自己識唔識啦!
Methoxybenzene when treated with a mixture of concentrated HNO3 and H2SO4 gives 4-methoxy-1-nitrobenzene as one of the products. Outline a reasonable mechanism for this reaction.”
2007年4月20日 星期五
會考化學(7)
titration(滴定):
1.1999 Paper I 7b
2. 2003 Paper I 8b
3. 2004 Paper I 7a
rate of reaction(反應速率):
1. 1994 Paper I 8
2. 2004 Paper I 8
electrolysis(電解):
1.2001 Paper I 9
2.2003 Paper I 7
2. 2005 Paper I 9
molar volume (摩爾體積):
1. 1998 Paper I 7b
2. 2000 Paper I 9a
3. 2002 Paper I 8a
化學友長氣咁講多次,記住做熟佢呀!!! 最後,記住做埋2005同埋2006嘅pastpaper,都有機返炒o架。下次化學友會同同學仔講下paragraphy-length question即係esaay呀。






